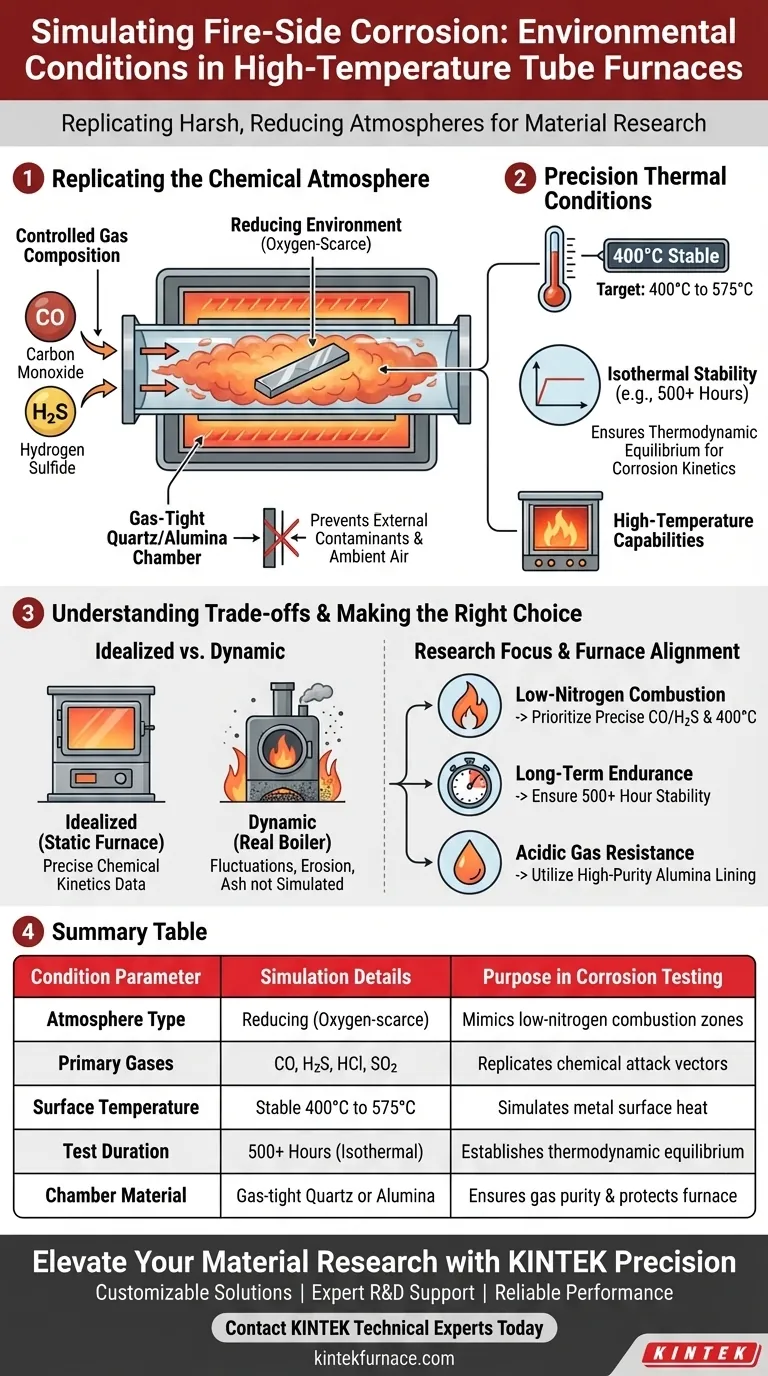
A high-temperature tube furnace primarily simulates the harsh, reducing atmospheres found in low-nitrogen combustion zones of power plant boilers. Specifically, these experiments create a controlled environment characterized by a stable metal surface temperature of 400°C and a precise mixture of corrosive gases, most notably Carbon Monoxide (CO) and Hydrogen Sulfide (H2S).
By isolating metal specimens in a gas-tight quartz or alumina chamber, these furnaces allow researchers to observe the exact corrosion kinetic behavior of steel without the variables found in an active boiler.

Replicating the Chemical Atmosphere
The Reducing Environment
The primary function of this setup is to mimic a reducing atmosphere. Unlike standard oxidation tests involving air, this environment replicates zones where oxygen is scarce, commonly found in low-nitrogen combustion areas.
Controlled Gas Composition
To simulate the specific chemical attack vectors of a fire, the furnace introduces specific ratios of mixed gases.
The primary reference highlights the use of Carbon Monoxide (CO) and Hydrogen Sulfide (H2S). These gases are introduced into the quartz reaction tube to study how they interact with steel surfaces to drive corrosion.
Protection from External Contaminants
To ensure the atmosphere remains pure, the reaction takes place inside gas-tight quartz or alumina tubes.
This isolation prevents ambient air from entering the chamber, ensuring the corrosion is caused solely by the introduced gas mixture and not by accidental oxidation.
Precision Thermal Conditions
Surface Temperature Simulation
The furnace is designed to maintain a specific target temperature for the metal specimen itself.
Based on the primary reference, a key benchmark for these experiments is maintaining a stable metal surface temperature of 400°C.
Isothermal Stability
Beyond just hitting a target temperature, the furnace ensures the environment is isothermal (constant temperature).
Supplementary data indicates that these systems can maintain thermal stability for extended periods (e.g., 500 hours), which is critical for establishing thermodynamic equilibrium.
High-Temperature Capabilities
While 400°C is a specific benchmark for certain steel specimens, these furnaces possess a broader range.
They are capable of facilitating reactions at higher bands, often between 500°C and 575°C, allowing for the study of various material limitations.
Understanding the Trade-offs
Idealized vs. Dynamic Conditions
While these furnaces provide excellent data on chemical kinetics, they create a static, idealized environment.
Real-world boilers experience rapid temperature fluctuations and physical erosion from ash, which a static tube furnace does not inherently simulate.
Chemical Aggression Risks
The gases used (H2S, HCl, SO2) are highly aggressive not just to the sample, but to the equipment.
Reliance on quartz or alumina linings is mandatory; without these chemically inert barriers, the corrosive gases would destroy the furnace body and invalidate the temperature controls.
Making the Right Choice for Your Goal
When designing a fire-side corrosion experiment, align your furnace settings with your specific research objectives.
- If your primary focus is reproducing low-nitrogen combustion zones: Prioritize the precise introduction of CO and H2S gases while maintaining a 400°C specimen temperature.
- If your primary focus is long-term material endurance: Ensure your furnace system is rated for long-duration stability (500+ hours) to allow for the complete formation and transformation of metal chlorides.
- If your primary focus is testing resistance to acidic gases: Utilize a furnace with a high-purity alumina lining to prevent equipment damage from HCl or SO2 exposure.
Precision in your environmental setup is the only way to transform raw data into actionable material insights.
Summary Table:
| Condition Parameter | Simulation Details | Purpose in Corrosion Testing |
|---|---|---|
| Atmosphere Type | Reducing (Oxygen-scarce) | Mimics low-nitrogen combustion zones |
| Primary Gases | CO, H2S, HCl, SO2 | Replicates chemical attack vectors of coal/fire |
| Surface Temperature | Stable 400°C to 575°C | Simulates metal surface heat in active boilers |
| Test Duration | 500+ Hours (Isothermal) | Establishes thermodynamic equilibrium for kinetics |
| Chamber Material | Gas-tight Quartz or Alumina | Ensures gas purity and protects furnace integrity |
Elevate Your Material Research with KINTEK Precision
Don’t leave your corrosion data to chance. KINTEK’s high-performance Tube, Muffle, and Vacuum furnace systems are engineered to provide the ultra-stable thermal environments and gas-tight integrity required for the most demanding fire-side corrosion experiments.
Why partner with KINTEK?
- Customizable Solutions: Whether you need alumina-lined chambers for acidic gas resistance or rotary systems for dynamic testing, we tailor our systems to your unique research needs.
- Expert R&D Support: Backed by industry-leading manufacturing, our furnaces ensure the isothermal stability required for 500+ hour endurance tests.
- Reliable Performance: Minimize downtime and maximize precision with equipment designed for harsh laboratory simulations.
Ready to transform your raw data into actionable material insights? Contact our technical experts today to find the perfect furnace solution for your laboratory.
Visual Guide
References
- Yifan Ni, Chenghao Fan. Investigating Fireside Corrosion Behavior and Mechanism of Low-Alloy Water Wall Tube of Ultra-Supercritical Power Plant. DOI: 10.3390/ma18071666
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What is the function of autoclaves and tube reactors in hydrometallurgical leaching? Unlock Refractory Ore Potential
- What role does a tube furnace play in the pyrolysis of covalent triazine frameworks? Optimize Your Carbon Synthesis
- What are the benefits of independent temperature control in a three-zone furnace? Enhance Precision and Uniformity
- Why is a high-temperature tubular furnace required for the activation process of walnut shell activated carbon at 700°C?
- What are the selection criteria for a quartz tube reactor used in RWGS testing? Optimize Your Catalyst Performance
- How do horizontal furnaces support the ceramics industry? Boost Performance with Precision Heat Treatment
- What advantages does a dual-zone tube furnace offer for carbon spheres? Enhanced Control & Superior Morphology
- What advantages does a drop tube furnace offer over other types of furnaces? Unlock Precision in Particle Thermal Analysis



















