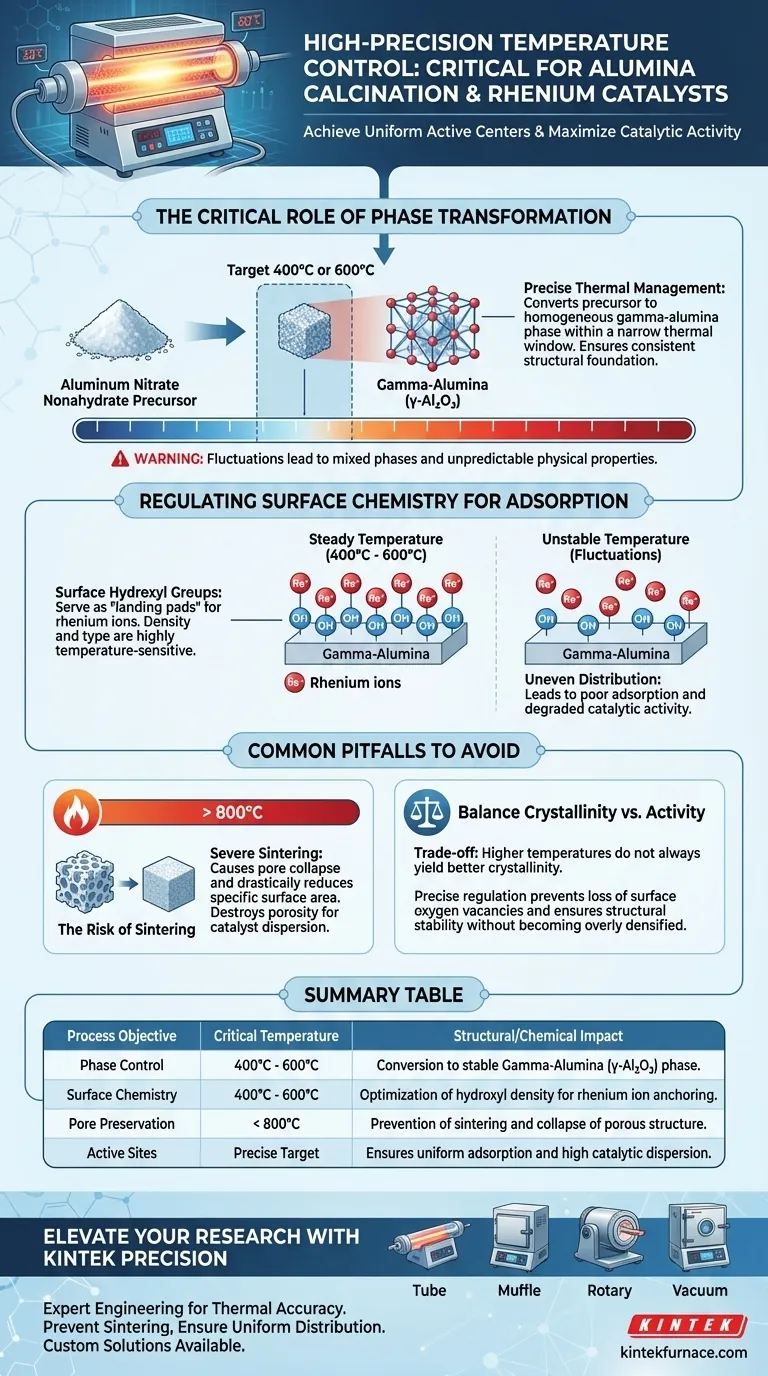
High-precision temperature control in a tube furnace is the defining factor in determining the ultimate performance of rhenium catalysts supported on alumina. When calcining precursors like aluminum nitrate nonahydrate, maintaining steady temperatures at specific targets—typically 400°C or 600°C—is essential to strictly control the alumina phase and the density of surface hydroxyl groups, which act as the anchoring sites for rhenium ions.
The thermal history of the support dictates its surface chemistry. Without precise temperature regulation, you cannot guarantee the consistent adsorption behavior required for a uniform distribution of active catalytic centers.

The Critical Role of Phase Transformation
Targeting the Gamma-Alumina Phase
The primary objective of calcination in this context is to convert the precursor into a specific crystalline phase, most notably gamma-alumina (gamma-Al2O3).
The transition from aluminum nitrate nonahydrate to gamma-alumina occurs within a narrow thermal window. Fluctuations in the tube furnace prevent the formation of a homogeneous phase, leading to structural inconsistencies in the support material.
Establishing the Structural Foundation
The physical structure of the support is "locked in" during this heating process. Precise thermal management ensures the material achieves the correct crystallinity without compromising its mechanical integrity.
Variations in heat can lead to mixed phases, which often possess different physical properties that react unpredictably during subsequent processing steps.
Regulating Surface Chemistry for Adsorption
Controlling Surface Hydroxyl Groups
The most nuanced aspect of this process is the regulation of surface hydroxyl groups. The density and type of these groups are highly sensitive to temperature.
These hydroxyl groups are not merely byproducts; they serve as the chemical "landing pads" for rhenium ions.
Impact on Rhenium Distribution
During the later impregnation process, the behavior of rhenium ions is directly dictated by the available surface hydroxyls.
If the temperature is too low or too high, the surface chemistry changes, leading to poor adsorption. This results in an uneven distribution of active centers, ultimately degrading the catalytic activity of the final product.
Common Pitfalls to Avoid
The Risk of Sintering
While distinct from the primary goal of creating gamma-alumina, it is crucial to understand the dangers of excessive heat. As noted in general calcination principles, temperatures approaching 800°C can lead to severe sintering.
Sintering causes the pore structure to collapse and drastically reduces the specific surface area. This physical degradation destroys the porosity needed for high catalyst dispersion.
Balancing Crystallinity and Activity
A common error is assuming that higher temperatures always yield better crystallinity. In reality, there is a trade-off between structural stability and surface activity.
Precise regulation prevents the loss of surface oxygen vacancies and ensures the material does not transition into an inactive or overly densified state.
Making the Right Choice for Your Goal
To optimize your rhenium catalyst preparation, align your thermal profile with your specific chemical targets:
- If your primary focus is Maximizing Rhenium Dispersion: Prioritize stability at 400°C or 600°C to optimize hydroxyl group density for uniform ion adsorption.
- If your primary focus is Structural Integrity: Ensure the furnace prevents temperature overshoots (e.g., toward 800°C) to avoid sintering and pore collapse.
Final Summary: The precision of your tube furnace is not just a process variable; it is the switch that controls the surface chemistry required to anchor rhenium effectively.
Summary Table:
| Process Objective | Critical Temperature | Structural/Chemical Impact |
|---|---|---|
| Phase Control | 400°C - 600°C | Conversion to stable Gamma-Alumina (γ-Al2O3) phase |
| Surface Chemistry | 400°C - 600°C | Optimization of hydroxyl density for rhenium ion anchoring |
| Pore Preservation | < 800°C | Prevention of sintering and collapse of porous structure |
| Active Sites | Precise Target | Ensures uniform adsorption and high catalytic dispersion |
Elevate Your Catalyst Research with KINTEK Precision
Achieving the perfect gamma-alumina phase and surface chemistry requires thermal accuracy that only expert engineering can provide. KINTEK empowers lab researchers and industrial manufacturers with high-performance Tube, Muffle, Rotary, and Vacuum furnaces designed for the most sensitive calcination processes.
Our systems offer the high-precision temperature regulation necessary to prevent sintering and ensure uniform rhenium distribution on your alumina supports. Whether you need a standard setup or a custom-engineered CVD system, our R&D team is ready to build a solution tailored to your unique catalytic needs.
Ready to optimize your thermal profiles? Contact KINTEK today for a customized furnace solution.
Visual Guide
References
- Joanna Malarz, Katarzyna Leszczyńska-Sejda. Research on the Production of Methyltrioxorhenium and Heterogenous Catalysts from Waste Materials. DOI: 10.3390/cryst15080717
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- Why is an atmosphere-controlled tube furnace used for La-CoTe2 synthesis? Master Your Tellurization Process Today
- How does a laboratory horizontal tube furnace facilitate the sintering of powder metallurgy structural steel?
- How does a laboratory tube furnace facilitate the sulfidation of Co3O4@CNT? Advanced Synthesis Secrets
- What components are in a turn-key quartz tube furnace? Essential parts for precise atmospheric control.
- What is the purpose of a high-purity argon protection system in a tube furnace? Safeguard MoS2/C Material Integrity
- Why is precise atmosphere control in a tube furnace critical for Ga2O3 annealing? Optimize Thin Film Defect Engineering
- How does the temperature zone layout of a horizontal tube furnace affect the synthesis quality of Bi2Se3 nanofilms?
- What are the benefits of integrating multiple heating zones in a tube furnace? Unlock Precise Thermal Control



















