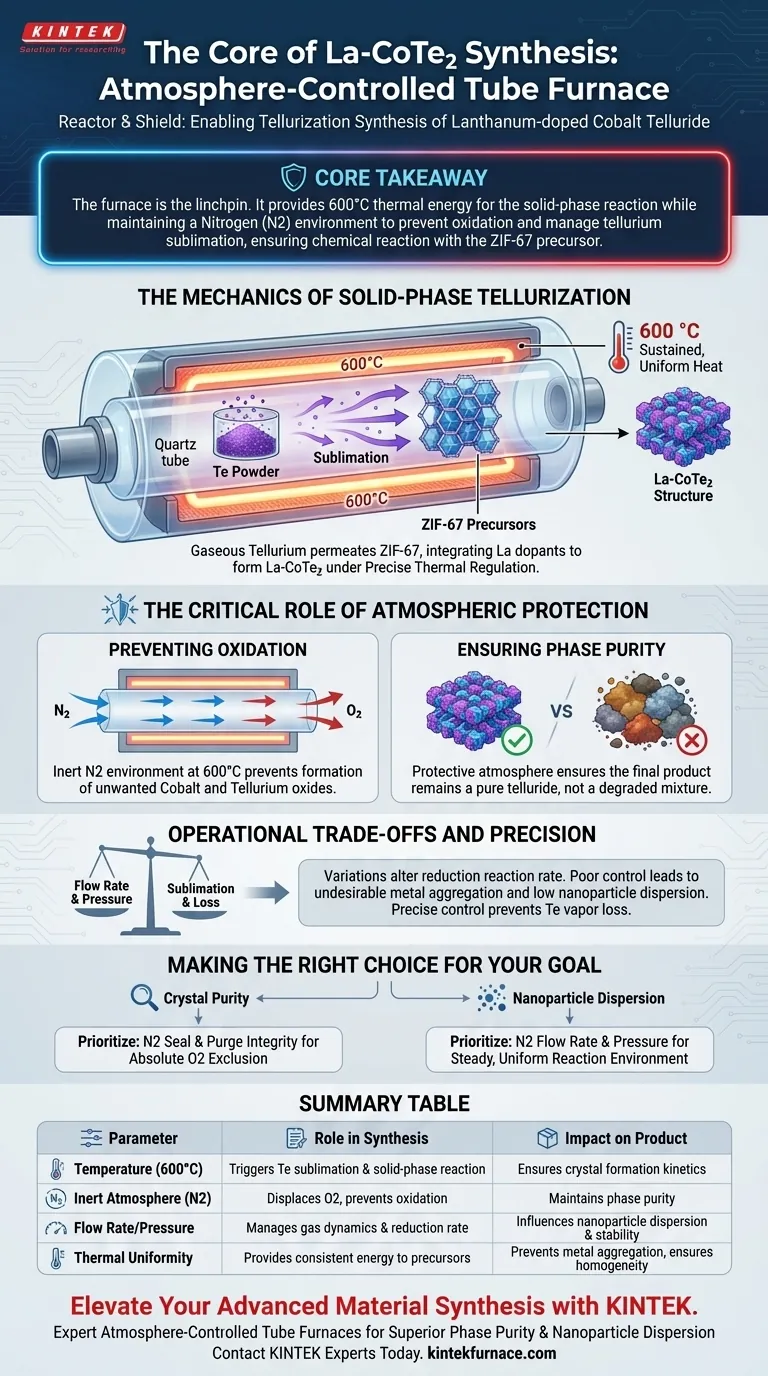
An atmosphere-controlled tube furnace is the linchpin of Lanthanum-doped Cobalt Telluride (La-CoTe2) synthesis because it acts as both a reactor and a shield. It provides the necessary thermal energy (600 °C) to trigger the solid-phase reaction while maintaining a nitrogen (N2) environment that strictly prevents oxidation and manages the sublimation of tellurium.
Core Takeaway The synthesis of La-CoTe2 relies on the sublimation of tellurium powder to penetrate a metal framework (ZIF-67). The tube furnace is critical because it isolates this process from oxygen, ensuring the tellurium reacts chemically with the precursor rather than burning off or degrading the cobalt.

The Mechanics of Solid-Phase Tellurization
The conversion of precursor materials into doped Cobalt Telluride is a delicate chemical process. The tube furnace provides the specific physical conditions required to drive this transformation.
Facilitating Tellurium Sublimation
For the reaction to occur, solid tellurium powder must be converted into a vapor phase. The furnace heats the materials to 600 °C, causing the tellurium to sublime.
Reaction with ZIF-67 Precursors
Once gaseous, the tellurium permeates the ZIF-67 (Cobalt-based metal-organic framework). This allows for a thorough chemical reaction with the metal framework, integrating the lanthanum dopants and forming the La-CoTe2 structure.
Precise Thermal Regulation
This process requires sustained, uniform heat. The tube furnace maintains the target temperature exactly, ensuring the reaction kinetics proceed at the correct rate for crystal formation.
The Critical Role of Atmospheric Protection
Beyond heating, the "atmosphere-controlled" aspect of the equipment is what safeguards the material's purity.
Preventing Oxidation
At 600 °C, cobalt and tellurium are highly reactive with oxygen. By continuously flushing the tube with nitrogen (N2), the furnace creates an inert environment that prevents the formation of unwanted oxides.
Ensuring Phase Purity
The specific La-CoTe2 crystal structure is sensitive to impurities. The protective atmosphere ensures that the final product remains a pure telluride rather than a degraded mixture of oxidized metals.
Operational Trade-offs and Precision
While the furnace enables the reaction, the quality of the output depends heavily on how the atmospheric parameters are managed.
The Impact of Flow Rate and Pressure
Simply filling the tube with nitrogen is not enough; the dynamics of the gas flow matter. Variations in flow rate and pressure can alter the reduction reaction rate, changing how the material forms.
Risks of Uneven Atmosphere
If the atmosphere is not distributed evenly, or if pressure fluctuates, the reaction becomes inconsistent. Poor control can lead to the undesirable aggregation of the metal phase, resulting in nanoparticles with low dispersion and poor stability.
Balancing Sublimation and Loss
There is a trade-off between ensuring enough tellurium sublimes to react and losing tellurium to the gas flow. Precise control prevents the tellurium vapor from being swept away before it can react with the ZIF-67 precursor.
Making the Right Choice for Your Goal
When configuring your tube furnace for La-CoTe2 synthesis, prioritize your settings based on the specific material properties you need to optimize.
- If your primary focus is Crystal Purity: Prioritize the integrity of the nitrogen seal and purge cycle to ensure absolute exclusion of oxygen before heating begins.
- If your primary focus is Nanoparticle Dispersion: Focus on optimizing the nitrogen flow rate and pressure to ensure a steady, uniform reaction environment that prevents metal aggregation.
Mastering the atmosphere within the tube is just as important as mastering the chemistry of the precursors.
Summary Table:
| Parameter | Role in La-CoTe2 Synthesis | Impact on Final Product |
|---|---|---|
| Temperature (600 °C) | Triggers Te sublimation and solid-phase reaction | Ensures kinetics for crystal formation |
| Inert Atmosphere (N2) | Displaces oxygen and prevents oxidation | Maintains phase purity and prevents degradation |
| Flow Rate/Pressure | Manages gas dynamics and reduction rate | Influences nanoparticle dispersion and stability |
| Thermal Uniformity | Provides consistent energy to ZIF-67 precursors | Prevents metal aggregation and ensures homogeneity |
Elevate Your Advanced Material Synthesis with KINTEK
Precision is non-negotiable when synthesizing complex materials like Lanthanum-doped Cobalt Telluride. KINTEK provides industry-leading, atmosphere-controlled tube furnaces designed to deliver the exact thermal stability and inert environment your research demands.
Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you are optimizing tellurization or developing new catalysts, our lab high-temp furnaces are fully customizable to meet your unique experimental needs.
Ready to achieve superior phase purity and nanoparticle dispersion?
Contact KINTEK Experts Today to find the perfect thermal solution for your laboratory.
Visual Guide
References
- Haonan Xie, Ting Deng. Reversible Sodium Storage of CoTe2 Anode via Lanthanum Doping. DOI: 10.3390/inorganics13060207
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What role does a tube furnace play in the pyrolysis of covalent triazine frameworks? Optimize Your Carbon Synthesis
- What role do industrial tube furnaces play in the oxidation of NiCrAl alloys? Precise Stability for Reliable Data
- What are the main components of a 70mm tube furnace? Uncover Key Parts for Precise Thermal Processing
- What are the common applications of quartz tube furnaces? Unlock Precision in High-Temp Processing
- Why is the vertical orientation of a drop tube furnace significant? Unlock Superior Process Control and Efficiency
- What is the use of a quartz tube furnace? For High-Purity, Observable Material Processing
- How does a split tube furnace compare to non-split tube furnaces? Choose the Right Furnace for Your Lab
- What role does a horizontal tube furnace play in chromite reduction? Master Precision Solid-State Processing



















