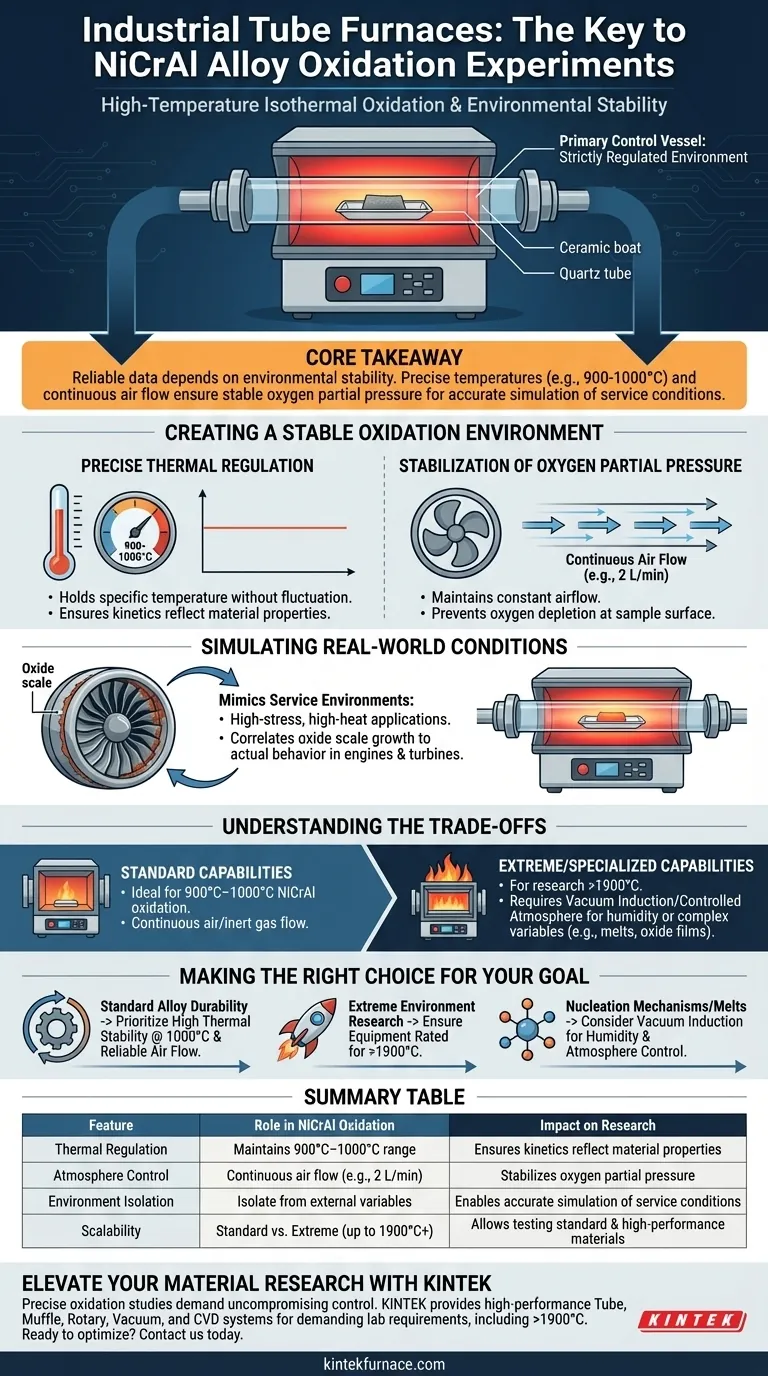
Industrial tube furnaces serve as the primary control vessel for high-temperature isothermal oxidation experiments on NiCrAl alloys. They provide a strictly regulated environment that isolates the sample, ensuring that the thermal conditions and gas atmosphere remain constant throughout the testing period.
Core Takeaway Reliable oxidation data depends entirely on environmental stability. By maintaining precise temperatures (such as 900°C or 1000°C) and a continuous air flow, industrial tube furnaces ensure stable oxygen partial pressure, allowing researchers to accurately simulate and predict how alloys will perform in actual service conditions.

Creating a Stable Oxidation Environment
To study how NiCrAl alloys degrade over time, researchers must eliminate environmental variables. Industrial tube furnaces achieve this through two primary mechanisms.
Precise Thermal Regulation
The fundamental requirement for isothermal experiments is holding a specific temperature without fluctuation.
Tube furnaces are engineered to maintain exact target temperatures, typically around 900°C to 1000°C for these specific alloy studies. This consistency ensures that the oxidation kinetics observed are a result of material properties, not thermal cycling or instability.
Stabilization of Oxygen Partial Pressure
Oxidation is a chemical reaction that consumes oxygen. If the air surrounding the sample becomes stagnant, the local oxygen levels drop, skewing the results.
These furnaces address this by maintaining a constant air flow rate, such as 2 L/min. This continuous flow ensures that the oxygen partial pressure remains stable and consistent at the sample surface throughout the entire reaction.
Simulating Real-World Conditions
The ultimate goal of these experiments is not just to burn metal, but to predict industrial performance.
Mimicking Service Environments
NiCrAl alloys are often used in high-stress, high-heat applications.
By strictly controlling the atmosphere and temperature, the tube furnace creates a laboratory model of these actual service conditions. This allows engineers to observe the growth of oxide scales in a way that correlates directly to how the material will behave in engines, turbines, or industrial processing equipment.
Understanding the Trade-offs
While industrial tube furnaces are ideal for standard oxidation studies, different research goals may require specialized equipment.
Standard vs. Extreme Capabilities
The standard tube furnace is excellent for the 900°C–1000°C range required for typical NiCrAl oxidation.
However, for research into high-performance material fabrication or extreme environments, standard units may fall short. Specialized furnaces are required for processes demanding temperatures exceeding 1900°C, pushing beyond the standard operating range of general oxidation experiments.
Atmosphere Limitations
Standard tube furnaces typically utilize a continuous flow of air or inert gas.
If your research requires precise regulation of humidity or vacuum conditions—such as studying oxide film growth on melts or specific transitions of amorphous alumina to crystalline structures—you may require laboratory-grade vacuum induction or controlled atmosphere furnaces rather than a standard industrial tube furnace.
Making the Right Choice for Your Goal
The selection of furnace equipment dictates the accuracy of your simulation.
- If your primary focus is standard alloy durability: Prioritize a furnace with high thermal stability at 1000°C and reliable air flow control to simulate typical service oxidation.
- If your primary focus is extreme environment research: Ensure your equipment is rated for temperatures exceeding 1900°C to handle high-performance fabrication limits.
- If your primary focus is nucleation mechanisms or melts: Consider vacuum induction furnaces that allow for precise control over humidity and complex atmospheric variables.
Select the equipment that mirrors the specific stress factors your material will face in the real world.
Summary Table:
| Feature | Role in NiCrAl Oxidation Experiments | Impact on Research |
|---|---|---|
| Thermal Regulation | Maintains constant 900°C–1000°C range | Ensures kinetics reflect material properties, not fluctuations |
| Atmosphere Control | Continuous air flow (e.g., 2 L/min) | Stabilizes oxygen partial pressure at the sample surface |
| Environment Isolation | Isolate sample from external variables | Enables accurate simulation of real-world service conditions |
| Scalability | Standard vs. Extreme (up to 1900°C+) | Allows for testing both standard alloys and high-performance materials |
Elevate Your Material Research with KINTEK
Precise oxidation studies demand uncompromising environmental control. Backed by expert R&D and world-class manufacturing, KINTEK provides high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems tailored for the most demanding lab requirements. Whether you are simulating service conditions for NiCrAl alloys or pushing boundaries with temperatures exceeding 1900°C, our customizable high-temperature furnaces deliver the stability you need.
Ready to optimize your isothermal experiments? Contact us today to discuss your unique laboratory needs.
Visual Guide
References
- Wojciech J. Nowak, Timur Galiullin. Combined Effect of Cold Working and Al Content on Oxidation Behavior of Ni-Base Alloys at 900 °C and 1000 °C. DOI: 10.1007/s11661-025-07830-4
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why is a high-temperature tube furnace required for NiWO4 calcination? Achieving High-Performance Cathode Materials
- What are the different types of tube furnaces and their features? Choose the Right Furnace for Your Lab
- What are tube furnaces used for? Achieve Precise Thermal Processing & Atmosphere Control
- Why is a high-vacuum tube furnace necessary for TMD annealing? Protect Your Monolayers from Oxidative Ablation
- How is a three-zone furnace structured? Unlock Precision Heating for Your Lab
- Why are multi zone tube furnaces particularly useful for nanomaterial research? Unlock Precise Thermal Control for Advanced Synthesis
- What are the primary functions of a precision gas filtration device? Maximize Data Integrity in Drop Tube Furnaces
- What is the purpose of using a tube furnace for a second calcination at 750°C? Mastering Biochar Activation



















