The primary function of a high-purity argon protection system is to establish a strictly inert environment that eliminates oxygen during high-temperature processing. Specifically, during the heat treatment of Molybdenum Disulfide/Carbon (MoS2/C) composites, this system prevents the chemical degradation of the sulfide core and the physical loss of the carbon shell.
Core Takeaway
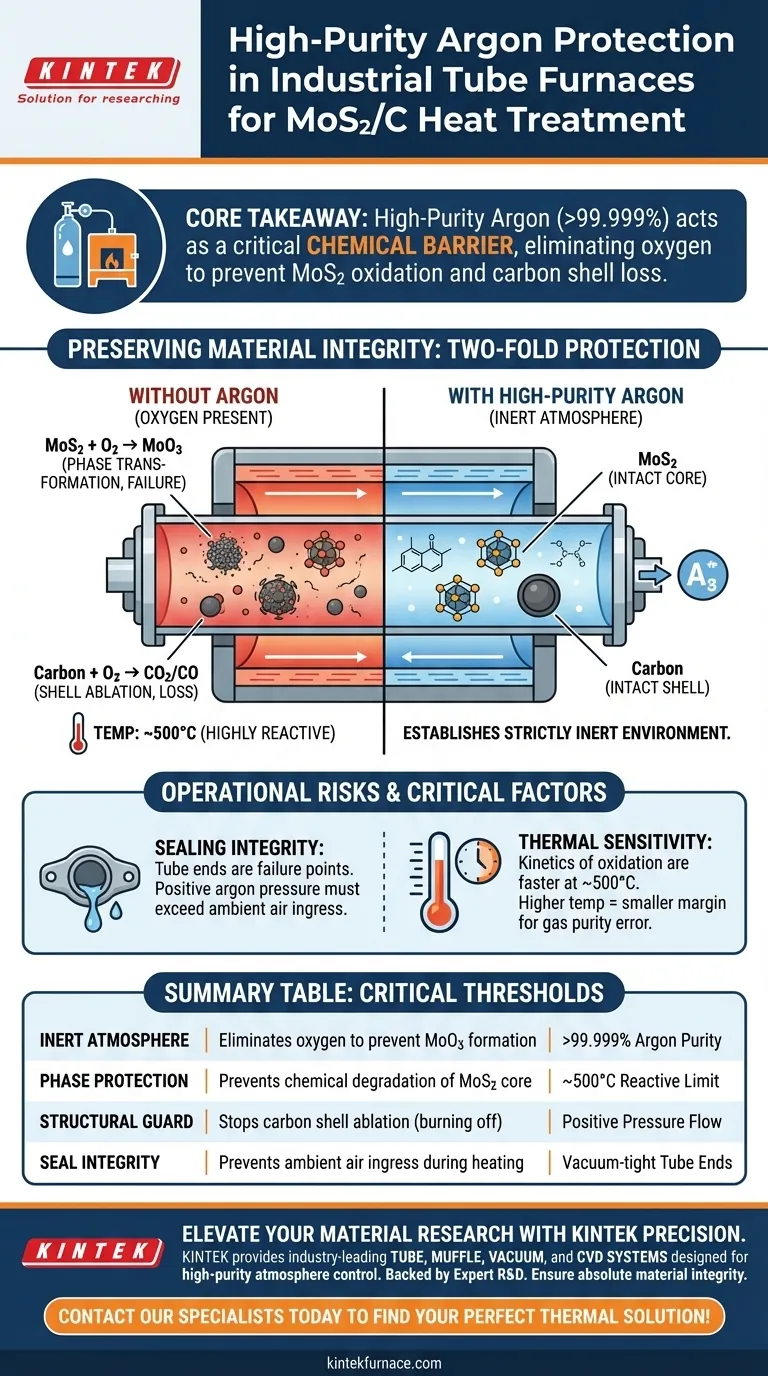
High-purity argon (exceeding 99.999%) acts as a critical chemical barrier, not just a thermal medium. Its sole purpose in this context is to prevent the oxidation of MoS2 into unwanted Molybdenum Trioxide (MoO3) and to stop the amorphous carbon shell from burning away, ensuring the composite's core-shell structure remains intact.
Preserving Material Integrity
The heat treatment of MoS2/C composites involves distinct chemical risks that dictate the need for a controlled atmosphere. The argon system addresses two specific failure modes that occur when temperatures rise.
Preventing Phase Transformation
At elevated temperatures, specifically around 500°C, Molybdenum Disulfide (MoS2) becomes highly reactive to oxygen.
Without an inert gas shield, MoS2 will react with oxygen to form Molybdenum Trioxide (MoO3). This is a fundamental change in the material's phase and properties, rendering the synthesis a failure if the goal is to maintain the sulfide structure.
Protecting the Carbon Shell
The "C" in MoS2/C refers to an amorphous carbon shell that encapsulates the core material.
During the carbonization process, this shell is vulnerable to ablation. If oxygen is present in the furnace chamber, the carbon will essentially burn off, converting into carbon dioxide or carbon monoxide gas. The argon blanket ensures this shell remains solid and continuous.
The Necessity of High Purity
Standard industrial argon is often insufficient for these applications.
The process requires high-purity argon (>99.999%). At high treatment temperatures, even trace amounts of impurities or oxygen in the gas flow can initiate the degradation reactions described above.
Operational Risks and Trade-offs
While the argon system is essential, it relies heavily on the mechanical integrity of the furnace itself. Understanding the limitations of the equipment is just as important as the gas purity.
Sealing Integrity vs. Gas Quality
You can utilize the purest argon available, but it is useless if the tube furnace has leaks.
Tube furnaces are designed to maintain these atmospheres, but the seals at the tube ends are common failure points. If the system is not perfectly sealed, the positive pressure of the argon flow must be high enough to prevent ambient air ingress.
Thermal Sensitivity
The reactions you are preventing are highly temperature-dependent.
While some inert atmosphere processes occur at lower temperatures (such as sulfur sublimation at 155°C), the MoS2/C process reaches roughly 500°C. At this higher thermal range, the kinetics of oxidation are much faster, making the margin for error regarding gas purity significantly smaller.
Making the Right Choice for Your Goal
When configuring your industrial tube furnace for composite materials, your setup should be dictated by the specific chemical vulnerabilities of your sample.
- If your primary focus is Phase Purity: Ensure your gas source is certified >99.999% argon to strictly prevent the conversion of MoS2 to MoO3.
- If your primary focus is Structural Morphology: Prioritize leak-checking the furnace seals to ensure the amorphous carbon shell is not lost to ablation during carbonization.
Success in this process is defined by the absolute absence of oxygen.
Summary Table:
| Feature | Function in MoS2/C Treatment | Critical Threshold |
|---|---|---|
| Inert Atmosphere | Eliminates oxygen to prevent MoO3 formation | >99.999% Argon Purity |
| Phase Protection | Prevents chemical degradation of MoS2 core | ~500°C Reactive Limit |
| Structural Guard | Stops carbon shell ablation (burning off) | Positive Pressure Flow |
| Seal Integrity | Prevents ambient air ingress during heating | Vacuum-tight Tube Ends |
Elevate Your Material Research with KINTEK Precision
Don't let trace oxygen compromise your MoS2/C synthesis. KINTEK provides industry-leading Tube, Muffle, Vacuum, and CVD systems specifically designed for high-purity atmosphere control. Backed by expert R&D and manufacturing, our high-temperature furnaces are fully customizable to meet your unique chemical and structural requirements.
Ensure absolute material integrity for your next project—contact our specialists today to find your perfect thermal solution!
Visual Guide
References
- One-Pot Hydrothermal Synthesis and Electrochemical Performance of Subspheroidal Core–Shell Structure MoS2/C Composite as Anode Material for Lithium-Ion Batteries. DOI: 10.3390/en17071678
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- How is the atmosphere controlled in a vacuum tube furnace? Achieve Precise Gas Environments for Your Experiments
- What is the function of a two-zone tube furnace in NiPS3 crystal growth? Master CVT for High-Quality Crystals
- What is the core function of a high-temperature tube furnace in converting Fe2O3/GO? Mastering Material Transformation
- What is the function of a quartz tube furnace during the growth of HA-CNT? Essential Guide for Precision CVD
- What are the dimensions and temperature capabilities of single zone horizontal tube furnace models? Explore Key Specs for Your Lab
- What is the function of a double-temperature zone tube furnace in CVD synthesis of 2D epsilon-Fe2O3 nanosheets?
- Why is a high-temperature tube furnace with an Argon atmosphere required for the carbonization of biomass? Key Insights
- How does an alumina-lined vertical tube furnace provide a stable environment for corrosion experiments? Get Expert Data



















