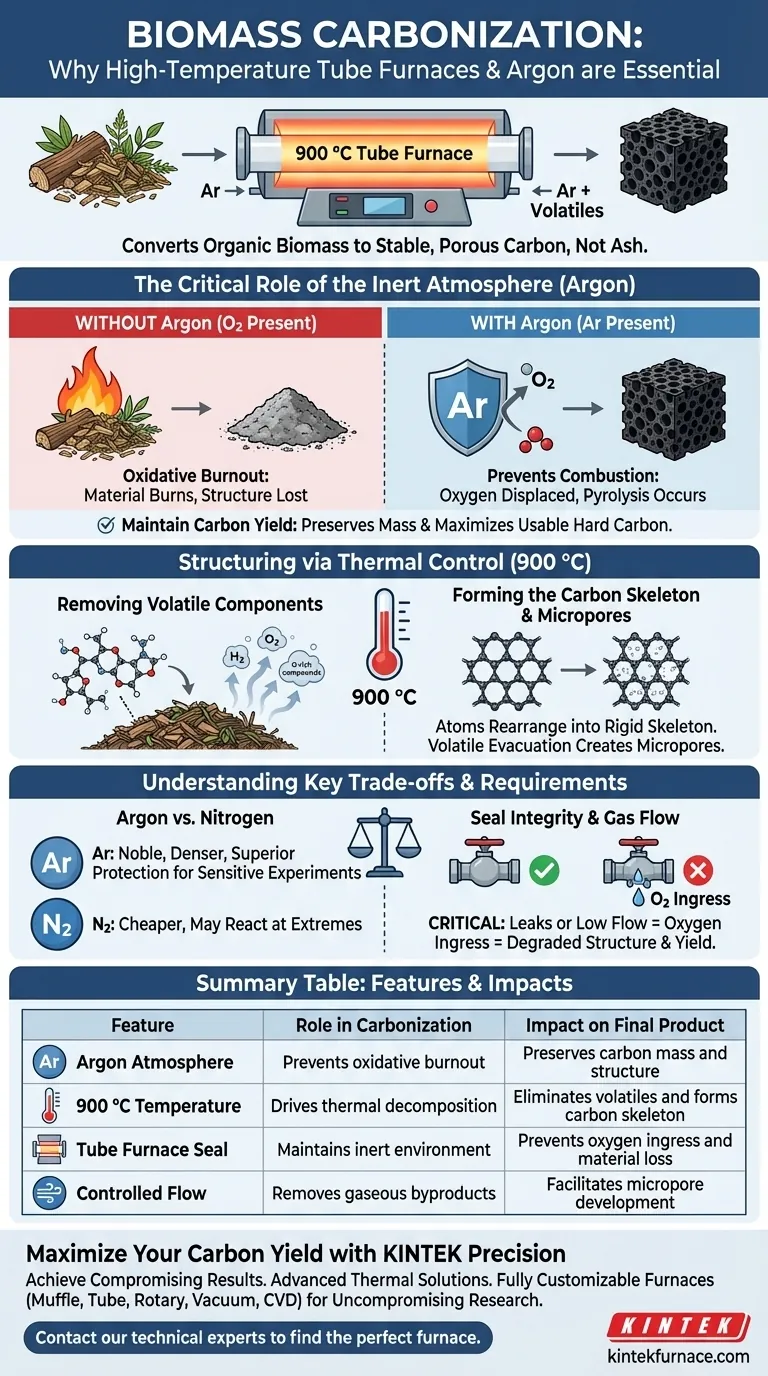
High-temperature tube furnaces with an Argon atmospheres are essential because they create the specific conditions needed to convert organic biomass into stable carbon rather than ash. At 900 °C, the Argon gas displaces oxygen to prevent combustion (oxidative burnout), while the furnace’s precise thermal control forces volatile components out, leaving behind a structured, porous carbon skeleton.
Carbonization requires a delicate balance: you must apply enough heat to strip away non-carbon elements, but you must strictly exclude oxygen to prevent the material from burning away completely.

The Critical Role of the Inert Atmosphere
Preventing Oxidative Burnout
The most immediate function of the Argon (Ar) atmosphere is the prevention of combustion.
If biomass is heated to 900 °C in the presence of air (oxygen), it simply burns, resulting in ash and a complete loss of the carbon structure.
Argon provides a strictly oxygen-free environment, ensuring the material undergoes pyrolysis (thermal decomposition) rather than oxidation.
Maintaining Carbon Yield
By preventing the carbon from reacting with oxygen, you preserve the mass of the material.
This protection is directly responsible for maintaining a high carbon yield, ensuring the maximum amount of biomass is converted into usable hard carbon.
Structuring the Material via Thermal Control
Removing Volatile Components
The tube furnace provides the necessary thermal energy to break down the organic precursors.
At temperatures around 900 °C, volatile components (such as hydrogen and oxygen-rich compounds) are vaporized and expelled from the material.
Forming the Carbon Skeleton
As volatiles are removed, the remaining atoms rearrange themselves.
This process transforms the initial polymer structure into a rigid, structurally stable carbon skeleton, which serves as the foundation for the material's physical strength.
Initiating Micropore Development
The evacuation of volatile gases leaves behind voids in the material matrix.
This initiates the development of a microporous structure, which is critical for applications requiring high surface area, such as adsorption or active component loading.
Understanding the Trade-offs
The Cost of Inert Gases
While Argon is highly effective, it is generally more expensive than Nitrogen, which is also used for inert atmospheres.
However, Argon is chemically noble and denser than air, often providing superior protection against oxidation in highly sensitive experiments where Nitrogen might react at extreme temperatures.
Sensitivity to Gas Flow and Sealing
The effectiveness of this process is entirely dependent on the integrity of the tube furnace's seal and gas flow management.
Even a minor leak or insufficient Argon flow rate allows oxygen ingress, which will degrade the porous structure and drastically reduce the final yield, regardless of the furnace temperature.
Making the Right Choice for Your Goal
To optimize your carbonization process, align your equipment settings with your specific objectives:
- If your primary focus is Structural Integrity and Yield: Prioritize a high-purity Argon flow and ensure the furnace seals are perfect to strictly prevent "burnout" and preserve the carbon skeleton.
- If your primary focus is Chemical Doping (e.g., Sulfur or Nitrogen): Focus on the precise temperature control (500-900 °C) and heating rates, as these parameters dictate how heteroatoms bond within the carbon framework.
Ultimately, the tube furnace and Argon atmosphere act as a controlled vacuum, stripping away the unnecessary to reveal the valuable carbon structure hidden within the biomass.
Summary Table:
| Feature | Role in Carbonization | Impact on Final Product |
|---|---|---|
| Argon Atmosphere | Prevents oxidative burnout | Preserves carbon mass and structure |
| 900 °C Temperature | Drives thermal decomposition | Eliminates volatiles and forms carbon skeleton |
| Tube Furnace Seal | Maintains inert environment | Prevents oxygen ingress and material loss |
| Controlled Flow | Removes gaseous byproducts | Facilitates micropore development |
Maximize Your Carbon Yield with KINTEK Precision
Achieve uncompromising results in your biomass research with KINTEK’s advanced thermal solutions. Backed by expert R&D and world-class manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your specific atmospheric and temperature requirements. Whether you are scaling up production or conducting sensitive lab-scale experiments, our furnaces provide the precise thermal control and airtight integrity needed to prevent burnout and optimize porous structures.
Ready to elevate your material synthesis? Contact our technical experts today to find the perfect furnace for your unique needs.
Visual Guide
References
- Himanshu Gupta, Debasish Sarkar. Bitter Apple Pulp‐Derived Porous Carbon with Rich Oxygen Functionalities for High‐Performance Zinc‐Ion Storage. DOI: 10.1002/smll.202502071
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What are some primary applications of the 70mm tube furnace? Unlock Precision in Materials Research
- What role does a tube furnace play in producing activated carbon? Master Walnut Shell Activation for High Adsorption
- Why must catalysts undergo reduction in a tube furnace? Master Your Furfural Hydrogenation Preparation
- What are the disadvantages of a tube furnace? Key Limitations for Industrial and Lab Use
- How does a tube muffle furnace contribute to the carbonization process of Rosa roxburghii residue biochar?
- What are the main applications of horizontal tube furnaces? Achieve Precise Heat Treatment and Synthesis
- What role does a horizontal tube furnace play in the carbonization of SiC-C preforms? Optimize Material Structural Yield
- What is the specific role of a Tube Furnace in phosphate/graphene annealing? Unlock High-Performance Electrode Synthesis



















