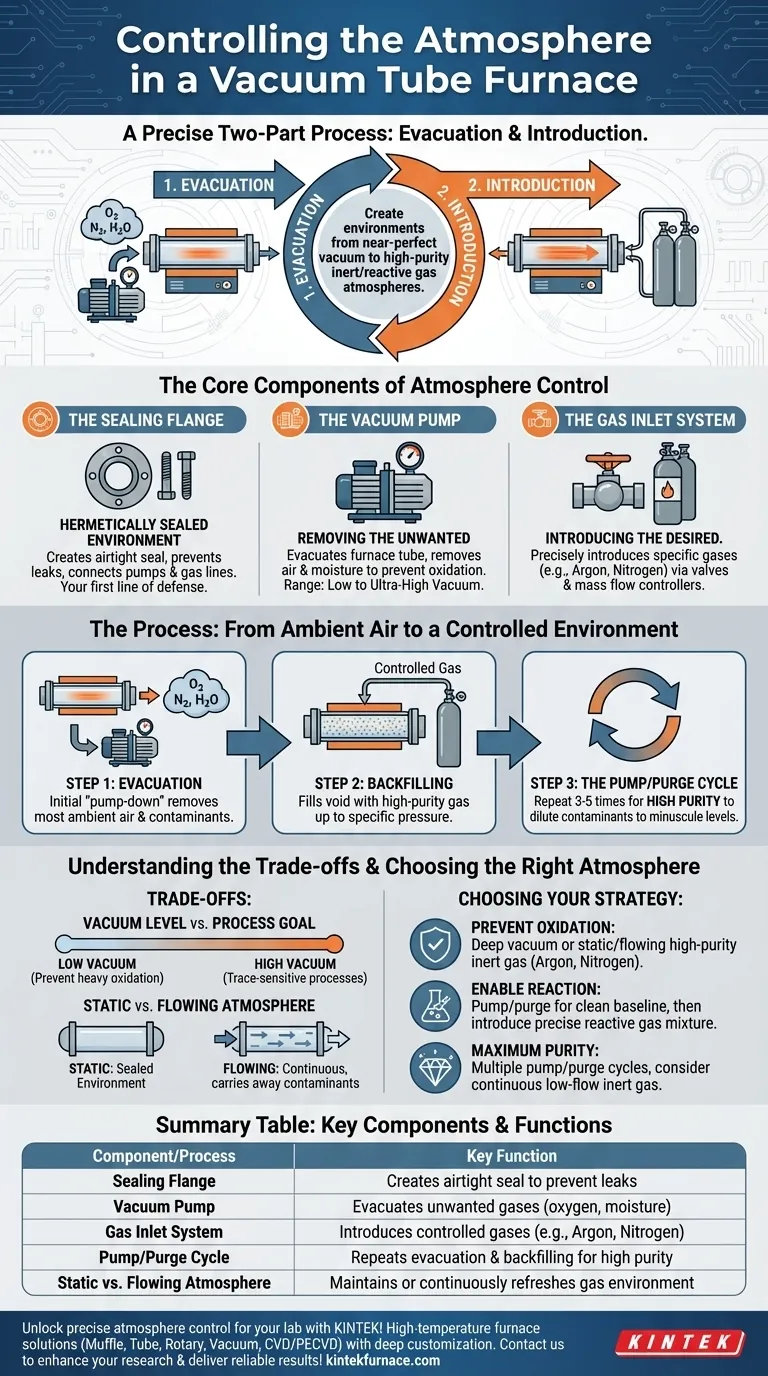
Controlling the atmosphere in a vacuum tube furnace is a precise two-part process. It is achieved by first evacuating unwanted ambient gases like oxygen with a vacuum pump, and then, if needed, introducing a specific, controlled gas or gas mixture through dedicated inlets. This dual capability allows for creating environments ranging from a near-perfect vacuum to a high-purity inert or reactive gas atmosphere.
The key to effective atmosphere control is not simply filling the tube with a new gas. It is the systematic removal of the existing, unwanted atmosphere first—a process known as purging—to ensure the final environment inside the furnace is as pure as your experiment demands.

The Core Components of Atmosphere Control
Achieving a controlled atmosphere is dependent on three critical hardware systems working in unison. Each plays a distinct role in sealing the chamber, removing gases, and introducing new ones.
The Sealing Flange: Your First Line of Defense
The entire process begins with a hermetically sealed environment. This is accomplished using stainless steel sealing flanges, which clamp onto the ends of the furnace tube.
These flanges are precision-engineered to create an airtight seal, preventing ambient air from leaking into the tube during operation. They are also equipped with the necessary ports for vacuum pumps and gas lines.
The Vacuum Pump: Removing the Unwanted
The vacuum pump is the heart of atmosphere control. Its job is to evacuate the furnace tube, removing the air and moisture that were present at the start.
This step is critical for preventing unwanted chemical reactions, primarily oxidation, which can compromise or destroy samples at high temperatures. The level of vacuum can range from low to ultra-high, depending on the pump system and the process requirements.
The Gas Inlet System: Introducing the Desired
Once a sufficient vacuum is achieved, a new atmosphere can be introduced. This is done through a gas inlet port on the flange, which is connected to one or more gas cylinders.
A system of valves and often a mass flow controller allows for the precise introduction of a specific gas, such as Argon or Nitrogen for an inert atmosphere, or a specific reactive gas for processes like chemical vapor deposition.
The Process: From Ambient Air to a Controlled Environment
The procedure for establishing the correct atmosphere is just as important as the hardware. Following a deliberate sequence ensures the highest level of purity.
Step 1: Evacuation (Creating the Vacuum)
The first step is always to run the vacuum pump to remove the ambient air from the sealed tube. This initial "pump-down" removes the vast majority of oxygen, nitrogen, and water vapor.
Step 2: Backfilling with a Controlled Gas
After evacuation, the tube is backfilled with the desired high-purity gas up to a specific pressure, which may be at or slightly above atmospheric pressure. This fills the void left by the evacuated air with the controlled gas.
Step 3: The Pump/Purge Cycle for High Purity
For optimal results, simply evacuating and backfilling once is often not enough. To achieve a truly pure atmosphere, the process should be repeated.
By evacuating the chamber, backfilling it with inert gas, and then evacuating it again, you dilute the remaining contaminants to minuscule levels. Repeating this pump/purge cycle three to five times is a common best practice for high-sensitivity applications.
Understanding the Trade-offs
Effective atmosphere control requires understanding the limitations and choices involved in the process.
Vacuum Level vs. Process Goal
Not every process requires an ultra-high vacuum. A low vacuum may be sufficient simply to prevent heavy oxidation. However, processes sensitive to trace amounts of oxygen or moisture will demand a high vacuum and rigorous purging cycles.
Static vs. Flowing Atmosphere
You can operate the furnace with a static atmosphere, where the tube is filled with gas and sealed. Alternatively, you can use a flowing atmosphere, where a small, continuous flow of gas enters one end of thetube and exits the other.
A flowing atmosphere is superior for carrying away outgassed contaminants from the sample or furnace walls during heating, ensuring a consistently pure environment throughout the process.
Interaction with Temperature and Cooling
The atmosphere is not isolated from the thermal cycle. Some processes use inert gas not just for protection, but also as a medium for forced cooling. By introducing a cool stream of inert gas after the heating phase, the sample can be cooled much faster than it would in a vacuum.
Choosing the Right Atmosphere for Your Process
Your experimental goal dictates your atmospheric strategy. Use these guidelines to make the right choice for your application.
- If your primary focus is preventing oxidation: A deep vacuum or a static/flowing atmosphere of high-purity inert gas like Argon or Nitrogen is the correct approach.
- If your primary focus is enabling a specific reaction: First, perform several pump/purge cycles with an inert gas to create a clean baseline, then introduce your precise reactive gas mixture.
- If your primary focus is achieving maximum purity: Always employ multiple pump/purge cycles before beginning your heat treatment, and consider using a continuous low-flow of inert gas throughout the process.
Mastering the control of your furnace's atmosphere is the key to unlocking repeatable, high-quality results.
Summary Table:
| Component/Process | Key Function |
|---|---|
| Sealing Flange | Creates airtight seal to prevent leaks |
| Vacuum Pump | Evacuates unwanted gases like oxygen and moisture |
| Gas Inlet System | Introduces controlled gases (e.g., Argon, Nitrogen) |
| Pump/Purge Cycle | Repeats evacuation and backfilling for high purity |
| Static vs. Flowing Atmosphere | Maintains or continuously refreshes gas environment |
Unlock precise atmosphere control for your lab with KINTEK! Leveraging exceptional R&D and in-house manufacturing, we provide advanced high-temperature furnace solutions like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we meet your unique experimental needs. Contact us today to discuss how our furnaces can enhance your research and deliver reliable results!
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why is a tube furnace utilized for the heat treatment of S/C composite cathode materials? Optimize Battery Stability
- What safety and reliability features are incorporated into a vertical tube furnace? Ensuring Safe, Consistent High-Temp Processing
- What role does a laboratory tube furnace perform during the carbonization of LCNSs? Achieve 83.8% Efficiency
- What is an example of a material prepared using a tube furnace? Master Precise Material Synthesis
- How is a high-temperature tube furnace utilized in the synthesis of MoO2/MWCNTs nanocomposites? Precision Guide



















