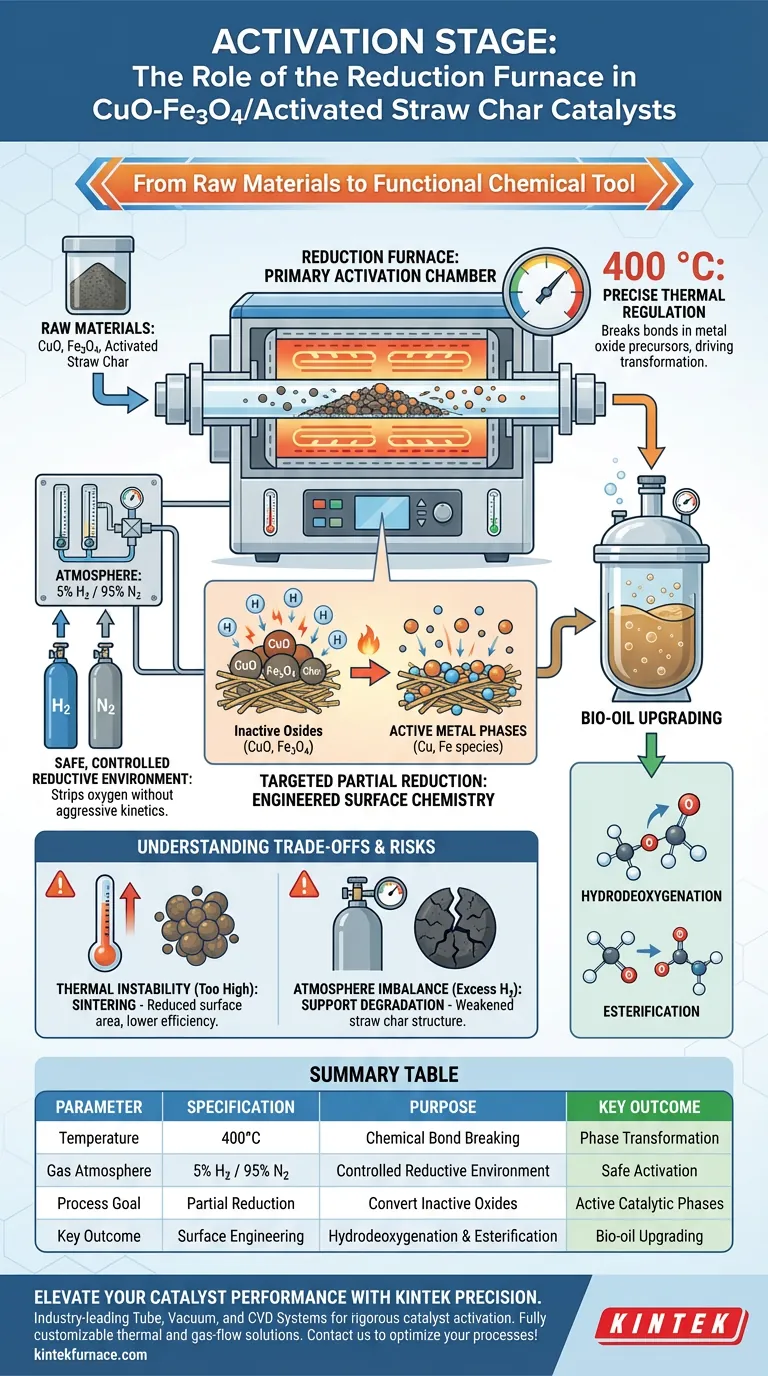
The reduction furnace functions as the primary activation chamber for CuO-Fe3O4/Activated Straw Char catalysts, bridging the gap between raw materials and a functional chemical tool.
It provides a strictly controlled environment—typically maintaining 400°C with a steady flow of mixed hydrogen and nitrogen (usually 5% H2)—to facilitate the partial reduction of metal oxide precursors. This thermal and chemical treatment converts inactive oxides into active metal phases, unlocking the specific catalytic properties required for bio-oil upgrading.
By subjecting the catalyst to a precise high-temperature reductive atmosphere, the furnace engineers the surface chemistry necessary for critical reactions like hydrodeoxygenation and esterification in supercritical ethanol environments.
The Mechanics of Catalyst Activation
Creating the Reductive Atmosphere
The furnace is responsible for maintaining a stable, flowing atmosphere of mixed gases, specifically hydrogen and nitrogen.
The primary reference indicates a typical concentration of 5% Hydrogen (H2). This specific ratio is critical because it provides the reducing agent (hydrogen) needed to strip oxygen atoms from the metal precursors without the safety risks or aggressive reaction kinetics associated with pure hydrogen.
Precise Thermal Regulation
Temperature control is the engine of the activation process. The furnace must maintain a constant temperature of 400°C.
At this specific thermal plateau, the energy is sufficient to break chemical bonds in the metal oxide precursors loaded on the straw char. This drives the transformation from a static oxide state into a chemically active phase capable of facilitating reactions.
Targeted Partial Reduction
The goal of this furnace operation is partial reduction, not necessarily complete metallization.
By controlling the exposure time and temperature, the furnace ensures the metal oxides (CuO and Fe3O4) are modified just enough to form active metal phases. This specific surface structure is what empowers the catalyst to perform hydrodeoxygenation (removing oxygen) and esterification (forming esters), which are essential for upgrading bio-oil quality.
Understanding the Trade-offs
The Risk of Thermal Instability
While the target is 400°C, deviations in the furnace's heating rate or holding temperature can compromise the catalyst.
If temperatures spike too high (instantaneous high heat), there is a risk of sintering, where the active metal particles clump together. This reduces the surface area and effectively kills the catalyst's efficiency before it is ever used.
Atmosphere Balance
The balance of hydrogen is a delicate trade-off between reactivity and structural integrity.
Insufficient hydrogen flow leads to incomplete activation, leaving the catalyst unable to perform bio-oil upgrading. Conversely, an uncontrolled reductive atmosphere could potentially degrade the activated straw char support, weakening the physical structure that holds the metal particles in place.
Making the Right Choice for Your Goal
To ensure your CuO-Fe3O4/Activated Straw Char catalyst performs effectively in supercritical ethanol environments, consider the following:
- If your primary focus is Catalytic Activity: Prioritize the precision of the 400°C temperature hold; deviations here will directly alter the hydrodeoxygenation capability.
- If your primary focus is Safety and Stability: strictly monitor the 5% H2/N2 gas mixture to ensure a controlled partial reduction without damaging the straw char support.
Success depends on using the reduction furnace not just as a heater, but as a precision tool to engineer specific active sites on the catalyst surface.
Summary Table:
| Parameter | Specification | Purpose |
|---|---|---|
| Temperature | 400°C | Facilitates chemical bond breaking for phase transformation |
| Gas Atmosphere | 5% H2 / 95% N2 | Provides safe, controlled reductive environment |
| Process Goal | Partial Reduction | Converts inactive oxides into active catalytic phases |
| Key Outcome | Surface Engineering | Enables hydrodeoxygenation and esterification capabilities |
Elevate Your Catalyst Performance with KINTEK Precision
Don't let thermal instability or inconsistent atmospheres compromise your catalyst's efficiency. KINTEK provides industry-leading Tube, Vacuum, and CVD systems designed for the rigorous demands of catalyst activation and chemical research.
Backed by expert R&D and manufacturing, our high-temperature furnaces are fully customizable to meet your specific thermal plateau and gas-flow requirements. Whether you are performing bio-oil upgrading or complex material synthesis, KINTEK ensures uniform heating and precision control every time.
Ready to optimize your lab's high-temperature processes? Contact us today to find your custom furnace solution!
Visual Guide
References
- Alhassan Ibrahim, El Barbary Hassan. Catalytic Upgrading of Rice Straw Bio-Oil via Esterification in Supercritical Ethanol over Bimetallic Catalyst Supported on Rice Straw Biochar. DOI: 10.3390/en17020407
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Heat Treat Sintering and Brazing Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What are the core tasks of vacuum and temperature systems in CSS for CZT films? Essential Control for High Performance
- What are the advantages of using a vacuum carburizing furnace? Achieve Superior Quality and Efficiency
- What is the core function of a vacuum distillation furnace in high-hazard waste magnesium recovery processes? Purify and Recycle Valuable Metal Safely
- What are the advantages of vacuum furnaces? Achieve Pristine Material Processing and Control
- Why is alloy composition more uniform when melted under vacuum or protective atmospheres? Ensure Precise Alloy Quality
- What technical requirements must a furnace meet for Inconel 718 hardening? Master Precision Aging & Cooling
- What are vacuum furnaces used for? Achieve Unmatched Material Purity and Performance
- Why does magnesium distillation use a two-stage pump? A strategic division of labor for efficiency.



















