In the synthesis of biochar via pyrolysis, the high-temperature ceramic crucible functions as a specialized vessel designed to ensure process integrity under extreme conditions. It acts as a chemically inert barrier that physically contains the biomass while withstanding temperatures up to 1000 °C without degrading.
The ceramic crucible serves as a guarantor of purity. By isolating the biomass in a thermally stable, non-reactive environment, it ensures that the chemical composition of the final biochar is determined solely by thermal decomposition, not by contamination from the container.

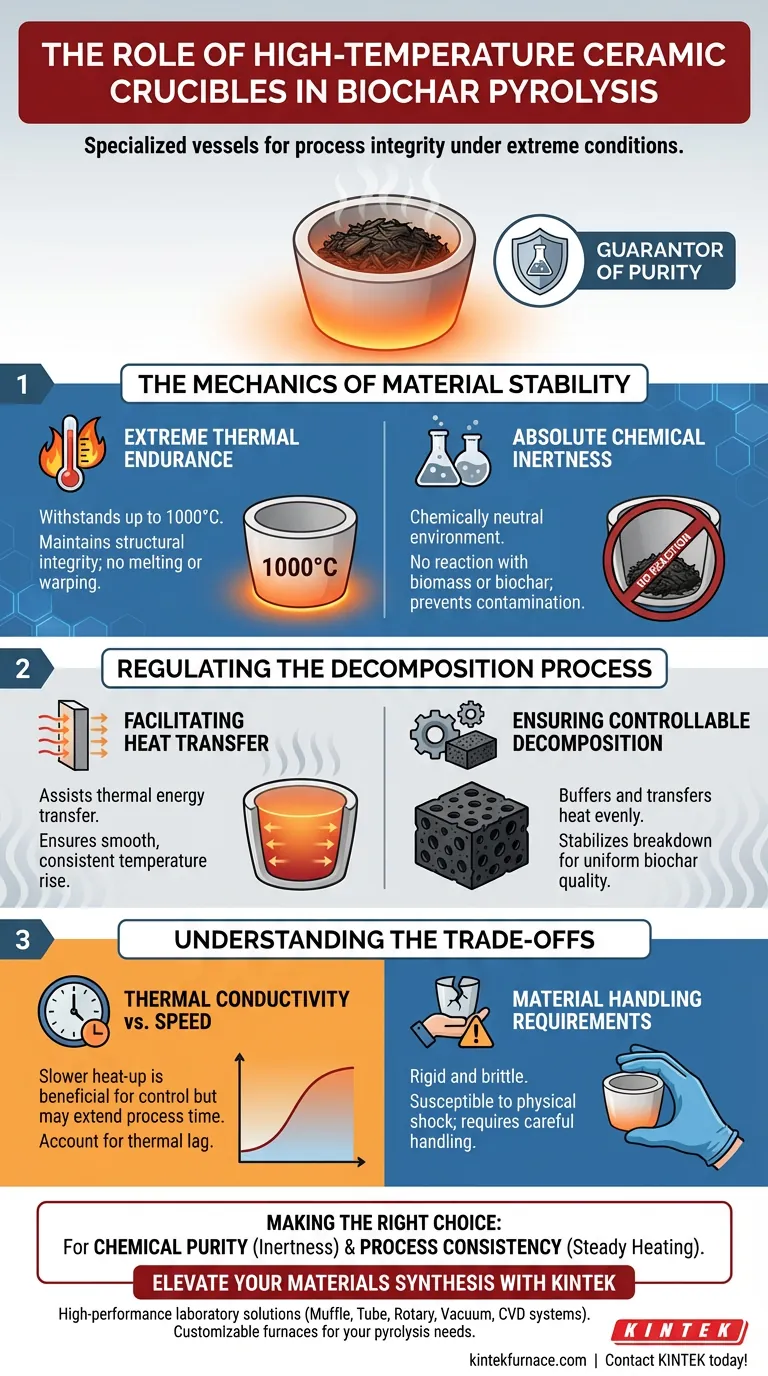
The Mechanics of Material Stability
Extreme Thermal Endurance
Pyrolysis requires subjecting biomass to intense heat to drive off volatiles and carbonize the remaining material. A ceramic crucible is selected specifically for its superior thermal stability.
It maintains its structural integrity at temperatures up to 1000 °C. This prevents the vessel from melting, warping, or failing during the critical high-heat phases of synthesis.
Absolute Chemical Inertness
The chemical environment inside a pyrolysis chamber is reactive, but the container must remain neutral. The ceramic material provides chemical inertness.
This ensures there is no reaction between the crucible and the raw biomass or the resulting biochar. This property is essential for accurate research and high-purity production, as it eliminates the risk of heavy metal leaching or catalytic effects from the vessel walls.
Regulating the Decomposition Process
Facilitating Heat Transfer
Beyond simple containment, the physical properties of the crucible play an active role in the thermodynamics of the process. The crucible possesses specific heat conduction properties.
These properties assist in transferring thermal energy from the furnace to the biomass sample. This conduction is critical for achieving a smooth, consistent rise in temperature throughout the sample material.
Ensuring Controllable Decomposition
Inconsistent heating can lead to incomplete carbonization or uneven biochar quality. The ceramic crucible aids in controllable biomass decomposition.
By buffering the heat and transferring it evenly, the crucible helps stabilize the rate at which the biomass breaks down. This results in a more uniform final product with predictable characteristics.
Understanding the Trade-offs
Thermal Conductivity vs. Speed
While the reference highlights the crucible's role in "smooth" decomposition, it is important to understand the thermal physics of ceramics. Ceramics generally heat up more slowly than metals.
This slower heat transfer is beneficial for control but may extend the time required to reach the target temperature center of the biomass. This "lag" must be accounted for when programming temperature ramps to ensure the core of the sample reaches the desired pyrolysis temperature.
Material Handling Requirements
The definition of a ceramic implies a rigid, brittle structure. While thermally stable, these crucibles lack the ductility of metal containers.
They are susceptible to physical shock or cracking if handled roughly, particularly when moving them in and out of the furnace. Users must prioritize careful handling to maintain the integrity of the containment vessel over repeated cycles.
Making the Right Choice for Your Goal
To maximize the quality of your biochar, you must align your equipment choices with your experimental objectives.
- If your primary focus is Chemical Purity: Rely on the ceramic crucible’s inertness to prevent elemental contamination, ensuring your biochar analysis reflects only the biomass feedstock.
- If your primary focus is Process Consistency: Utilize the heat conduction properties of the ceramic to maintain a steady temperature ramp, preventing thermal spikes that could alter the biochar's pore structure.
By leveraging the stability and inertness of high-temperature ceramics, you transform the pyrolysis vessel from a simple container into a precision instrument for quality control.
Summary Table:
| Feature | Role in Pyrolysis | Key Benefit |
|---|---|---|
| Chemical Inertness | Prevents reactions between vessel and biomass | Ensures high-purity biochar without contamination |
| Thermal Endurance | Withstands temperatures up to 1000°C | Maintains structural integrity during high-heat cycles |
| Controlled Conduction | Buffers and transfers heat to the sample | Promotes uniform carbonization and predictable pore structure |
| Physical Isolation | Contains feedstock in a stable environment | Protects process integrity from external furnace elements |
Elevate Your Materials Synthesis with KINTEK
Precision in biochar production starts with the right thermal environment. KINTEK provides high-performance laboratory solutions backed by expert R&D and manufacturing. Whether you need Muffle, Tube, Rotary, Vacuum, or CVD systems, our high-temp furnaces are fully customizable to meet your unique pyrolysis requirements.
Maximize your lab's efficiency and ensure sample purity—Contact KINTEK today to discuss your custom furnace needs!
Visual Guide
References
- Fairuz Gianirfan Nugroho, Abu Talha Aqueel Ahmed. Utilizing Indonesian Empty Palm Fruit Bunches: Biochar Synthesis via Temperatures Dependent Pyrolysis. DOI: 10.3390/nano15010050
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant Rotating Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What should be evaluated when assessing supplier reliability for alumina ceramic furnace tubes? Ensure Consistent Performance and Support
- What other industrial applications do graphite crucible furnaces have beyond metal melting? Unlock Advanced Material Processing
- How do Mass Flow Controllers (MFC) contribute to the repeatability of In2Se3 synthesis? Master CVD Process Stability
- What are the functions of high-purity, high-strength graphite molds in SPS? Optimize Al2O3-TiC Ceramic Sintering
- What materials are commonly used for furnace tubes to withstand high heat? Choose the Best for Your Lab
- What role does a quartz substrate holder play in MoS2 growth? Optimize Thin Film Deposition with Precision Hardware
- How does a laboratory vacuum pump system contribute to the preparation process of TixNbMoTaW refractory alloys?
- Why use sealed quartz tubes & vacuum for Mg-Zn/Mg-Cd alloy prep? Ensure Compositional Purity


















