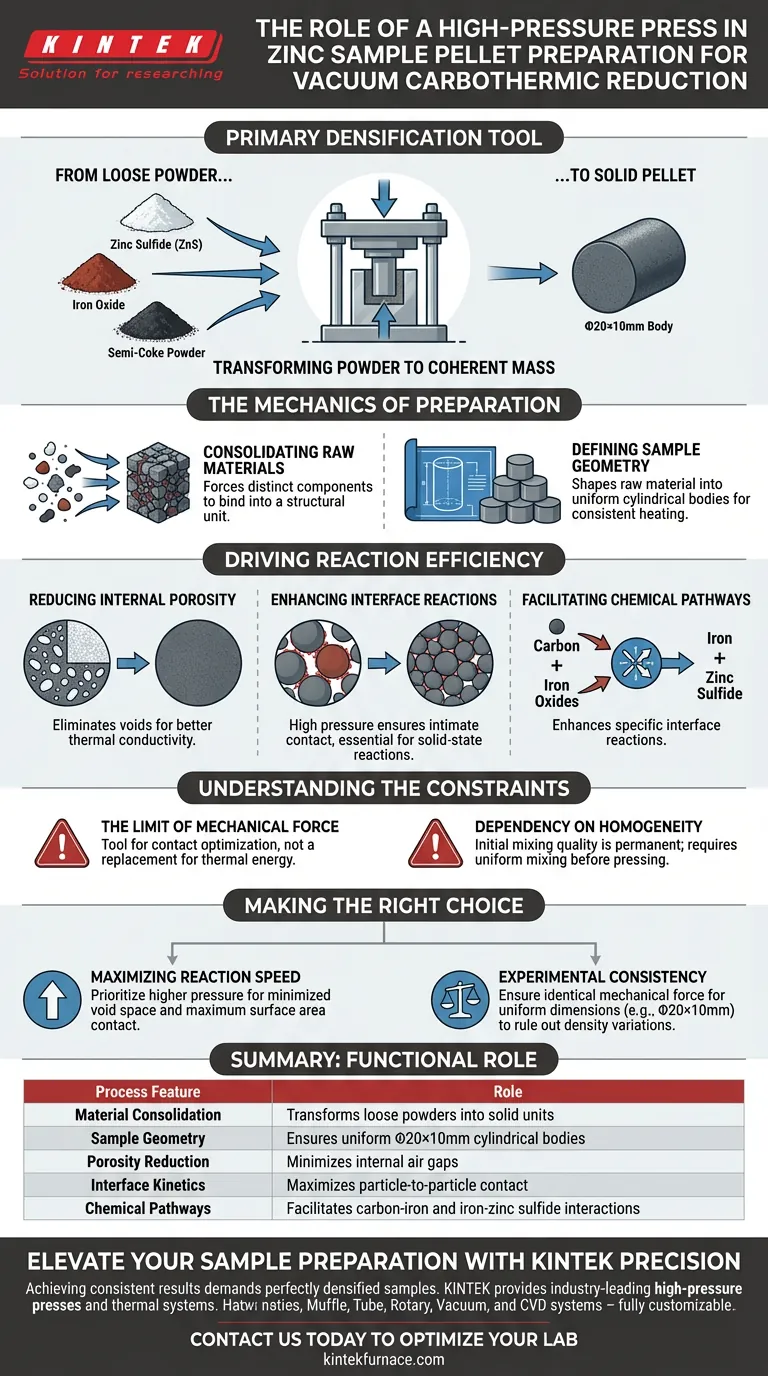
A high-pressure press functions as the primary densification tool in the preparation of zinc samples, transforming loose reactant powders into a solid, coherent mass. Its immediate purpose is to compact a mixture of zinc sulfide (ZnS), iron oxide, and semi-coke powder into specific cylindrical geometries, such as Φ20×10mm bodies, to prepare them for the vacuum furnace.
By applying significant mechanical force, the press minimizes internal porosity and maximizes the surface area contact between particles. This physical proximity is the fundamental requirement for driving efficient interface reactions in the solid-state reduction process.

The Mechanics of Sample Preparation
Consolidating Raw Materials
The process begins with a loose mixture of raw ingredients, specifically zinc sulfide, iron oxide, and semi-coke powder.
Without a press, these materials exist as separate particles with significant air gaps between them. The press forces these distinct components to bind together into a single structural unit.
Defining Sample Geometry
The press shapes the raw material into uniform cylindrical bodies.
Common dimensions for these samples are approximately Φ20×10mm. This uniformity is crucial for ensuring consistent heat distribution and reaction rates across different experimental trials or production runs.
Driving Reaction Efficiency
Reducing Internal Porosity
The most critical function of the high-pressure press is the reduction of internal porosity.
Loose powders contain a high volume of void space (air). By eliminating these voids, the press creates a dense medium that facilitates better thermal conductivity and material transport.
Enhancing Interface Reactions
In vacuum carbothermic reduction, reactions occur at the points where different particles touch.
The high pressure ensures that reactant particles are brought into intimate, close contact. This is not merely structural; it is chemical.
Facilitating Specific Chemical Pathways
The densification allows for specific interface reactions to occur more efficiently.
First, it enhances the reaction between carbon and iron oxides. Second, it improves the interaction between the resulting iron and the zinc sulfide. Without the dense contact provided by the press, these solid-solid reactions would be significantly slower and less efficient.
Understanding the Constraints
The Limit of Mechanical Force
While high pressure is beneficial, it is a tool for contact optimization, not a replacement for thermal energy.
The press prepares the "stage" for the reaction, but the vacuum and heat are still required to drive the chemical reduction.
Dependency on Homogeneity
The press locks the particles into place, meaning the initial mixing quality is permanent once the pellet is formed.
If the zinc sulfide, iron oxide, and semi-coke are not mixed uniformly before pressing, the high-pressure compaction will result in areas of poor reaction efficiency, regardless of how dense the pellet is.
Making the Right Choice for Your Goal
If your primary focus is Maximizing Reaction Speed: Prioritize higher pressure settings to minimize void space and maximize the direct contact surface area between carbon, iron, and zinc compounds.
If your primary focus is Experimental Consistency: Ensure that the mechanical force applied is identical for every cylindrical body (e.g., maintaining exact Φ20×10mm dimensions) to rule out density variations as a variable.
The high-pressure press effectively bridges the gap between raw potential and kinetic reality by forcing reactants close enough to interact on a molecular level.
Summary Table:
| Process Feature | Functional Role of High-Pressure Press |
|---|---|
| Material Consolidation | Transforms loose ZnS, iron oxide, and coke powders into solid units |
| Sample Geometry | Ensures uniform Φ20×10mm cylindrical bodies for consistent heating |
| Porosity Reduction | Minimizes internal air gaps to improve thermal conductivity |
| Interface Kinetics | Maximizes particle-to-particle contact for faster solid-state reactions |
| Chemical Pathways | Facilitates efficient carbon-iron and iron-zinc sulfide interactions |
Elevate Your Sample Preparation with KINTEK Precision
Achieving consistent results in vacuum carbothermic reduction requires more than just high temperatures—it demands perfectly densified samples. KINTEK provides industry-leading high-pressure presses and thermal systems designed to bridge the gap between raw powder and reaction-ready pellets.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of lab solutions, including Muffle, Tube, Rotary, Vacuum, and CVD systems, all of which are fully customizable to meet your unique metallurgical and chemical research needs.
Ready to optimize your lab's efficiency and experimental accuracy? Contact us today to find the perfect high-temperature furnace or pressing solution!
Visual Guide
References
- Hang Ma, Xixia Zhao. Iron oxide synergistic vacuum carbothermal extraction of zinc from zinc sulfide. DOI: 10.2298/jmmb231212024m
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Hot Press Furnace Machine Heated Vacuum Press
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- How have vacuum hot press furnaces transformed material processing? Achieve Superior Density and Purity
- How does a vacuum hot press sintering furnace mitigate copper sintering swelling? Solve Fe-Cu Expansion Issues
- What is the primary function of the vacuum environment in a vacuum hot press furnace during the sintering of graphite flake/copper composites? Ensure High-Performance Thermal Conductivity
- What medical applications benefit from Vacuum Hot Press technology? Enhance Biocompatible Implants and Tools
- What functions do graphite molds serve in the vacuum hot pressing of copper-carbon nanotube composites?
- Why must the surface layer of titanium alloy samples be removed by grinding? Ensure High Strength After Vacuum Hot Press
- How does hot-press sintering contribute to manufacturing high-density Ta-Al-C MAX phase ceramics? Optimize Consolidation
- What are the temperature-based classifications for vacuum hot pressing sintering furnaces? Choose the Right Furnace for Your Materials



















