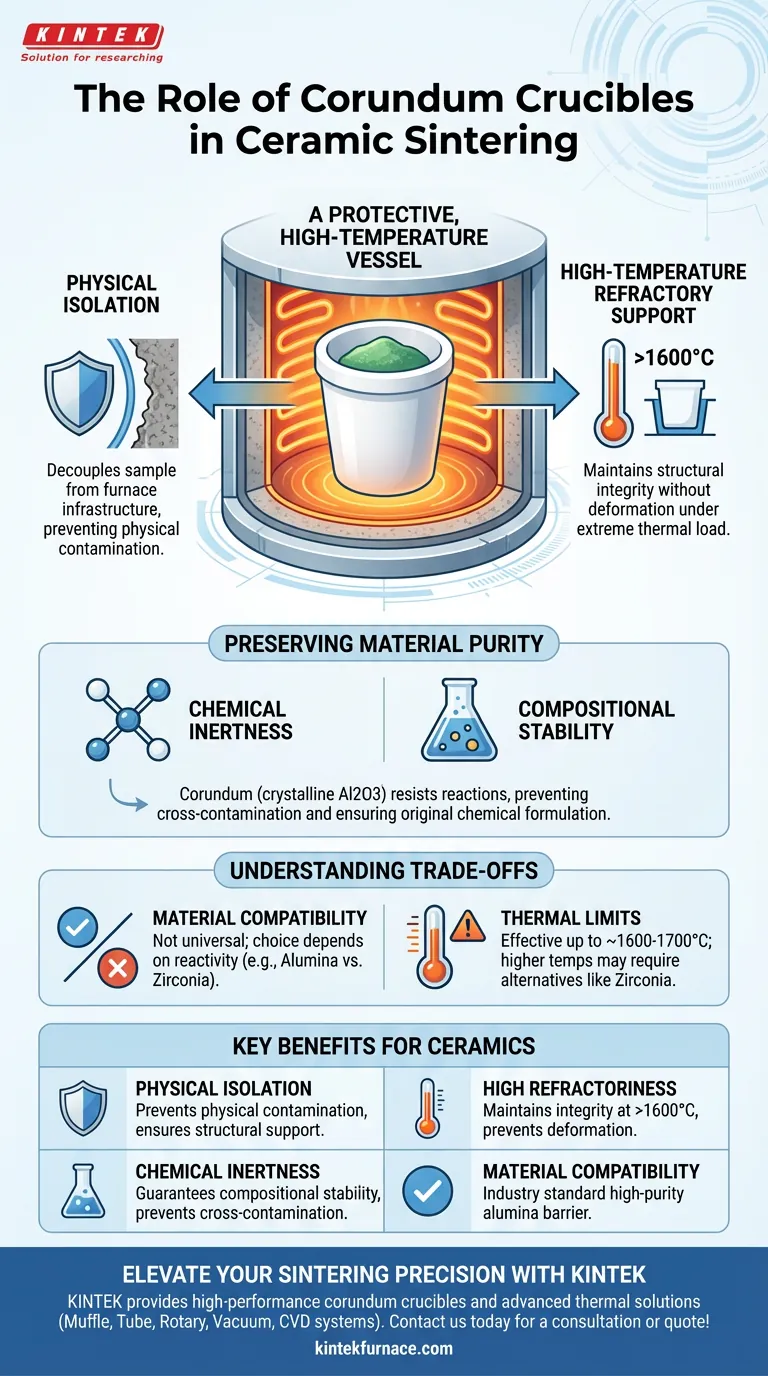
A corundum crucible serves as a protective, high-temperature vessel designed to physically support and chemically isolate ceramic samples during the sintering process. Its primary function is to act as a barrier, preventing the sample from coming into direct contact with furnace linings or heating elements while withstanding extreme thermal environments.
The core value of a corundum crucible lies in its ability to maintain a chemically inert environment at temperatures exceeding 1600°C, ensuring the final ceramic product retains its intended composition without contamination.
The Mechanics of Protection
Physical Isolation
The fundamental role of the crucible is to provide a stable physical boundary. Inside a furnace, heating elements and insulation materials can be sources of physical contamination.
By placing samples inside the crucible, you effectively decouple the sample from the furnace infrastructure. This ensures the material remains structurally supported throughout the heating cycle.
High-Temperature Refractory Support
Sintering requires intense heat to induce atomic diffusion. A corundum crucible is classified as a highly refractory container.
It is engineered to maintain its structural integrity at temperatures exceeding 1600 degrees Celsius. This allows it to hold samples securely without softening, deforming, or failing under thermal load.
Preserving Material Purity
Chemical Inertness
Beyond physical support, the crucible plays a vital chemical role. Corundum (crystalline aluminum oxide) is renowned for its excellent chemical inertness.
During the sintering process, materials become highly reactive. The corundum crucible resists reacting with the sample, preventing cross-contamination that would alter the material's properties.
Compositional Stability
For high-performance ceramics, precise chemical composition is critical. Any interaction between the vessel and the sample can introduce impurities.
Because the corundum crucible does not leach elements or react with the ceramic load, it guarantees that the sample maintains its original chemical formulation throughout the sintering process.
Understanding the Trade-offs
Material Compatibility
While corundum is an excellent general-purpose refractory material, it is not a universal solution for every element.
As noted in broader metallurgical contexts, the choice of crucible material—whether Alumina (corundum), Zirconia, or Graphite—depends heavily on the specific reactivity of the material being processed.
Thermal Limits
Corundum performs exceptionally well up to roughly 1600°C–1700°C. However, for processes requiring even higher temperatures or involving metals that react specifically with alumina, alternative refractory materials like Zirconia may be required.
Making the Right Choice for Your Process
To ensure the success of your sintering process, evaluate your requirements against the crucible's capabilities:
- If your primary focus is Compositional Purity: Rely on corundum crucibles to prevent chemical reactions between the sample and the container.
- If your primary focus is High-Temperature Stability: Use corundum for processes reaching up to 1600°C, ensuring the vessel provides rigid support without deformation.
- If your primary focus is Reactive Material Processing: Verify that your specific sample material does not have a chemical affinity for aluminum oxide before proceeding.
The corundum crucible is the industry standard for stabilizing the sintering environment, acting as the guardian of both the sample's shape and its chemistry.
Summary Table:
| Feature | Role in Sintering Process | Benefit for Ceramics |
|---|---|---|
| Physical Isolation | Decouples samples from furnace linings/heating elements | Prevents physical contamination and ensures structural support |
| High Refractoriness | Maintains integrity at temperatures >1600°C | Prevents vessel deformation or failure under extreme thermal load |
| Chemical Inertness | Resists reactions with reactive ceramic loads | Guarantees compositional stability and prevents cross-contamination |
| Material Compatibility | Acts as a high-purity alumina (Al2O3) barrier | Industry standard for preserving high-performance material properties |
Elevate Your Sintering Precision with KINTEK
Don't let contamination compromise your research or production. KINTEK provides high-performance corundum crucibles and advanced thermal solutions designed for the most demanding laboratory environments.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, along with a full range of lab high-temp furnaces—all customizable to meet your unique sintering needs. Whether you require superior chemical inertness or specialized thermal stability, our technical team is ready to assist you.
Ready to optimize your ceramic processing? Contact us today for a consultation or quote!
Visual Guide
References
- Wencke Mohring, Christiane Stephan‐Scherb. High-Temperature Corrosion of High- and Medium-Entropy Alloys CrMnFeCoNi and CrCoNi Exposed to a Multi-Oxidant Atmosphere H2O–O2–SO2. DOI: 10.1007/s44210-023-00026-8
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What are the reasons for using high-purity alumina material for the reaction tubes in a Drop Tube Furnace? - Guide
- How does a PID temperature controller facilitate gasification research? Unlock Precision in Industrial Simulations
- Why are quartz boat properties and cleanliness critical for Si:B nanowires? Ensure High-Purity Synthesis Success
- What are the primary functions of the vacuum pump system and inert gases? Achieve High-Purity Atomization
- Why Is a High-Precision Heating and Stirring Platform Necessary for ZnO Sol-Gel Synthesis? Achieve Perfect Nanoparticles
- What is the purpose of using a PID controller to drive a heating furnace? Master Thermal Kinetics Precision
- What is the water-saving benefit of using a water circulating vacuum pump? Save Over 10 Tons of Water Daily
- Why introduce argon flow into a steel crucible for ZK51A alloy? Ensure Safety and High-Purity Melting



















