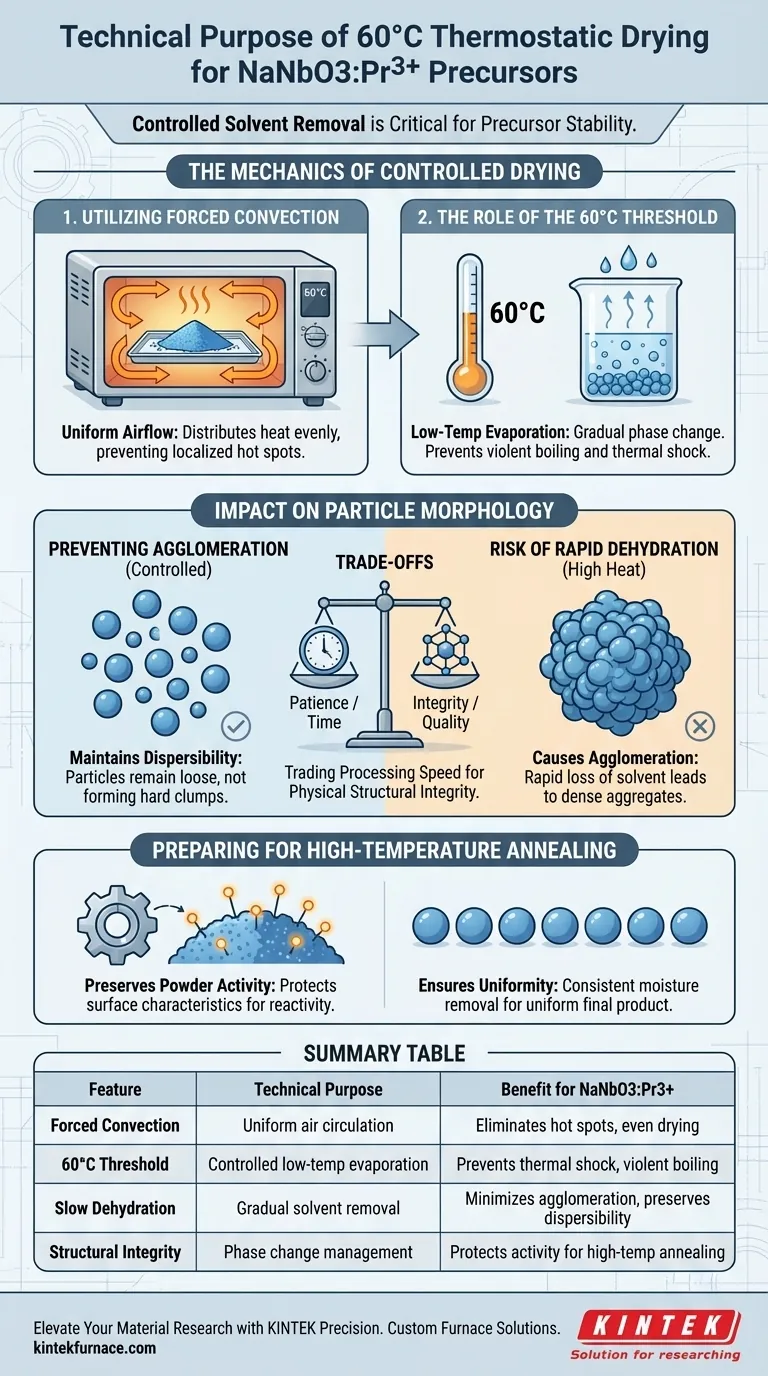
Controlled solvent removal is critical for precursor stability. The technical purpose of using an electric thermostatic drying oven at 60 °C is to uniformly eliminate moisture and residual solvents from NaNbO3:Pr3+ precursors using forced convection. This specific thermal treatment prevents the structural damage associated with rapid dehydration, ensuring the material remains physically suitable for subsequent processing.
By maintaining a controlled low-temperature environment, this process prevents particle agglomeration and preserves the powder's natural dispersibility. This step is foundational for maintaining the powder activity required to synthesize high-quality final products.

The Mechanics of Controlled Drying
Utilizing Forced Convection
The electric thermostatic oven operates on the principle of forced convection.
This mechanism circulates heated air continuously throughout the chamber.
The constant airflow ensures that heat is distributed evenly, preventing localized "hot spots" that could unevenly dry the precursor batch.
The Role of the 60 °C Threshold
Operating at 60 °C creates a distinct "low-temperature environment."
This temperature is sufficient to evaporate water and common solvents without inducing violent boiling or thermal shock.
It allows for a gradual phase change from liquid to vapor, which is less disruptive to the material's structure than high-heat drying.
Impact on Particle Morphology
Preventing Particle Agglomeration
The primary risk during the drying phase of NaNbO3:Pr3+ precursors is particle agglomeration.
If dehydration occurs too rapidly, particles tend to bind together tightly, forming hard clumps.
Controlled drying at 60 °C mitigates this, keeping the particles separate and preventing the formation of dense aggregates.
Maintaining Dispersibility
For the precursor to function correctly in later stages, it must maintain high dispersibility.
This means the particles should remain loose and capable of spreading evenly.
The thermostatic drying process preserves this physical characteristic, ensuring the powder does not fuse into an unworkable mass.
Preparing for High-Temperature Annealing
Preserving Powder Activity
The ultimate goal of the precursor is to undergo high-temperature annealing to form the final crystal structure.
To do this effectively, the powder must retain its chemical "activity" or reactivity.
Gentle drying protects surface characteristics that drive these reactions, ensuring the material responds correctly when heat is later increased.
Ensuring Uniformity
A uniform precursor leads to a uniform final product.
By removing solvents consistently across the entire batch, the oven ensures that every part of the sample enters the annealing phase in the same state.
Understanding the Trade-offs
The Cost of Patience
The primary trade-off of drying at 60 °C is time.
Because the temperature is relatively low, moisture removal is a slower process compared to high-heat methods.
You are effectively trading processing speed for physical structural integrity.
Risks of Rapid Dehydration
Attempting to speed up this process by increasing the temperature defeats the purpose of the precursor preparation.
Rapid dehydration causes the very agglomeration this step is designed to avoid.
Once particles have agglomerated due to high heat, it is often impossible to restore their dispersibility, permanently compromising the final product quality.
Making the Right Choice for Your Goal
To maximize the efficacy of your NaNbO3:Pr3+ synthesis, consider the following recommendations based on your objectives:
- If your primary focus is Final Product Quality: Prioritize the 60 °C forced convection cycle to maximize powder activity and minimize defects.
- If your primary focus is Process Consistency: Rely on the thermostatic control to ensure every batch enters the annealing stage with identical moisture content.
Strict adherence to this low-temperature drying protocol is the most effective way to guarantee a dispersible, high-activity precursor.
Summary Table:
| Feature | Technical Purpose | Benefit for NaNbO3:Pr3+ |
|---|---|---|
| Forced Convection | Uniform air circulation | Eliminates localized hot spots and ensures even drying |
| 60 °C Threshold | Controlled low-temp evaporation | Prevents thermal shock and violent boiling of solvents |
| Slow Dehydration | Gradual solvent removal | Minimizes particle agglomeration and preserves dispersibility |
| Structural Integrity | Phase change management | Protects surface activity for high-temperature annealing |
Elevate Your Material Research with KINTEK Precision
Achieving the perfect precursor state requires absolute thermal consistency. At KINTEK, we specialize in high-performance laboratory solutions tailored for advanced material synthesis. Backed by expert R&D and world-class manufacturing, we provide a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all of which are fully customizable to meet your unique drying and annealing specifications.
Don't let inconsistent heat compromise your powder activity. Partner with KINTEK to ensure every batch meets the highest standards of purity and dispersibility.
Contact our technical experts today to find your custom furnace solution!
Visual Guide
References
- Zhangnan WANG. Personalized Electronic Signature Technology Based on Stress Luminescent Materials. DOI: 10.5755/j02.ms.39962
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What are the advantages of HTL reactors for algae? Optimize Biomass Conversion Without Pre-Drying
- What is preventive maintenance on a furnace? A Proactive Strategy for Peak Performance
- What is zirconium dioxide and how is it stabilized for dental use? Discover the Science Behind Durable Dental Ceramics
- Why is a high-precision furnace critical for refractory castables? Ensure Structural Integrity & Mineral Stability
- Why is an industrial drying oven necessary for Boron Carbide mixed slurries? Ensure Coating Integrity & Precision
- What is the primary function of a high-precision drop furnace? Master Flash Smelting Simulation Kinetics
- What role does Thermogravimetric Analysis (TGA) play in determining the calcination parameters for manganese phosphate?
- What is the role of high-purity helium in electromagnetic levitation? Key for Rapid Thermal Regulation



















