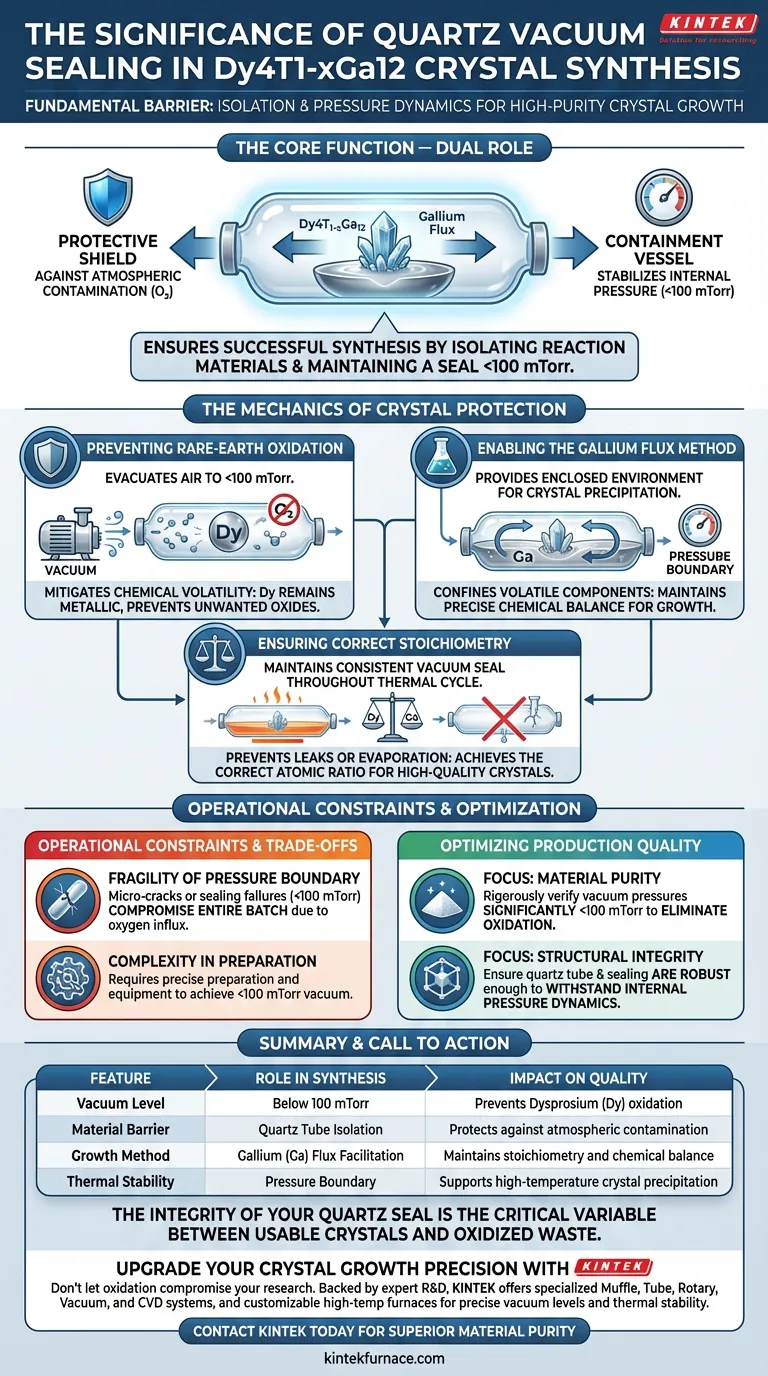
Quartz vacuum sealing technology is the fundamental barrier ensuring the successful synthesis of Dy4T1-xGa12 crystals. It functions by isolating reaction materials from the external environment and maintaining a seal under pressures below 100 mTorr. This specific vacuum environment effectively prevents the rapid oxidation of rare-earth Dysprosium (Dy) at elevated temperatures while creating the enclosed pressure dynamics necessary for the Gallium (Ga) flux method to function correctly.
The primary value of this technology lies in its dual role: it acts as a protective shield against atmospheric contamination and as a containment vessel that stabilizes the internal pressure required for high-purity, stoichiometric crystal growth.

The Mechanics of Crystal Protection
Preventing Rare-Earth Oxidation
The most immediate risk in producing Dy4T1-xGa12 is the chemical volatility of its components.
Rare-earth elements, specifically Dysprosium (Dy), are highly susceptible to oxidation when exposed to air at high temperatures.
Quartz vacuum sealing mitigates this risk by evacuating air to levels below 100 mTorr, ensuring the Dy remains metallic and reactive only with the intended components, rather than forming unwanted oxides.
Enabling the Gallium Flux Method
Beyond protection, the sealed quartz tube plays an active role in the growth mechanics.
The production of these crystals relies on the Gallium (Ga) flux method, which requires a specific, enclosed environment to facilitate crystal precipitation.
The quartz tube serves as a robust pressure boundary, confining the volatile components within a closed system to maintain the precise chemical balance needed for growth.
Ensuring Correct Stoichiometry
The ultimate goal of the process is achieving the correct atomic ratio, or stoichiometry.
Any leak or failure in isolation would alter the concentration of reactants through oxidation or evaporation.
By maintaining a consistent vacuum seal, the system ensures that the ratio of Dysprosium to Gallium remains constant throughout the thermal cycle, resulting in high-quality crystals.
Operational Constraints and Trade-offs
The Fragility of the Pressure Boundary
While quartz is an excellent material for thermal isolation, it introduces physical constraints.
The seal must remain intact under rigorous thermal stress; any micro-cracks or sealing failures will immediately break the vacuum (< 100 mTorr).
This loss of vacuum compromises the entire batch, as the influx of oxygen will degrade the rare-earth materials instantly.
Complexity in Preparation
Achieving a vacuum below 100 mTorr requires precise preparation and equipment.
This adds a layer of complexity to the manufacturing process compared to open-system methods.
However, for Dy4T1-xGa12, this trade-off is unavoidable, as open methods cannot support the necessary chemical stability for these specific materials.
Optimizing Production Quality
To maximize the yield and quality of your crystal growth, prioritize the following based on your specific objectives:
- If your primary focus is Material Purity: Rigorously verify that your vacuum system consistently achieves pressures significantly below 100 mTorr to completely eliminate oxidation risks for the Dysprosium.
- If your primary focus is Structural Integrity: Ensure the quartz tube wall thickness and sealing technique are robust enough to withstand the internal pressure dynamics of the Gallium flux at peak temperatures.
The integrity of your quartz seal is the single most critical variable in determining whether you produce a usable crystal or a sample of oxidized waste.
Summary Table:
| Feature | Role in Dy4T1-xGa12 Synthesis | Impact on Quality |
|---|---|---|
| Vacuum Level | Below 100 mTorr | Prevents Dysprosium (Dy) oxidation |
| Material Barrier | Quartz Tube Isolation | Protects against atmospheric contamination |
| Growth Method | Gallium (Ga) Flux Facilitation | Maintains stoichiometry and chemical balance |
| Thermal Stability | Pressure Boundary | Supports high-temperature crystal precipitation |
Upgrade Your Crystal Growth Precision with KINTEK
Don't let oxidation compromise your rare-earth material research. The integrity of your quartz seal is the difference between high-purity Dy4T1-xGa12 crystals and oxidized waste.
Backed by expert R&D and manufacturing, KINTEK offers specialized Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable lab high-temp furnaces designed to maintain precise vacuum levels and thermal stability. Whether you are scaling production or refining material stoichiometry, our high-performance solutions are tailored to your unique lab needs.
Ready to achieve superior material purity? Contact KINTEK today to discuss your project requirements!
Visual Guide
References
- S. Lee, Daniel C. Fredrickson. Interstitial Atoms and the Frustrated and Allowed Structural Transitions Principle: Tunability in the Electronic Structure of AuCu<sub>3</sub>‐type Frameworks in Dy<sub>4</sub>T<sub>1−<i>x</i></sub>Ga<sub>12</sub> (T = Ag, Ir). DOI: 10.1002/zaac.202500079
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What are the primary functions of a quartz tube reactor? Enhance Hydrogen Production and Induction Efficiency
- What are the advantages of using borosilicate glass for the upper atmosphere control chamber? Protect Your Vacuum Seals
- Is there a need to add water when launching the circulating water multifunctional vacuum pump? Ensure Optimal Performance and Avoid Damage
- What role do vacuum-sealed high-purity silica ampoules play in phase equilibrium experiments? Enhance Sample Integrity
- Why use a high-purity alumina crucible with a lid for LATP sintering? Ensure Optimal Stoichiometric Stability
- Why is radiation correction necessary for K-type thermocouple readings? Ensure Accurate High-Temp Combustion Data
- What is the primary role of laboratory furnaces in manufacturing and scientific processes? Unlock Precision Thermal Control
- How do recirculating coolant baths and glass bottles improve CHP? Boost Bio-oil Yield with Precision Cooling



















