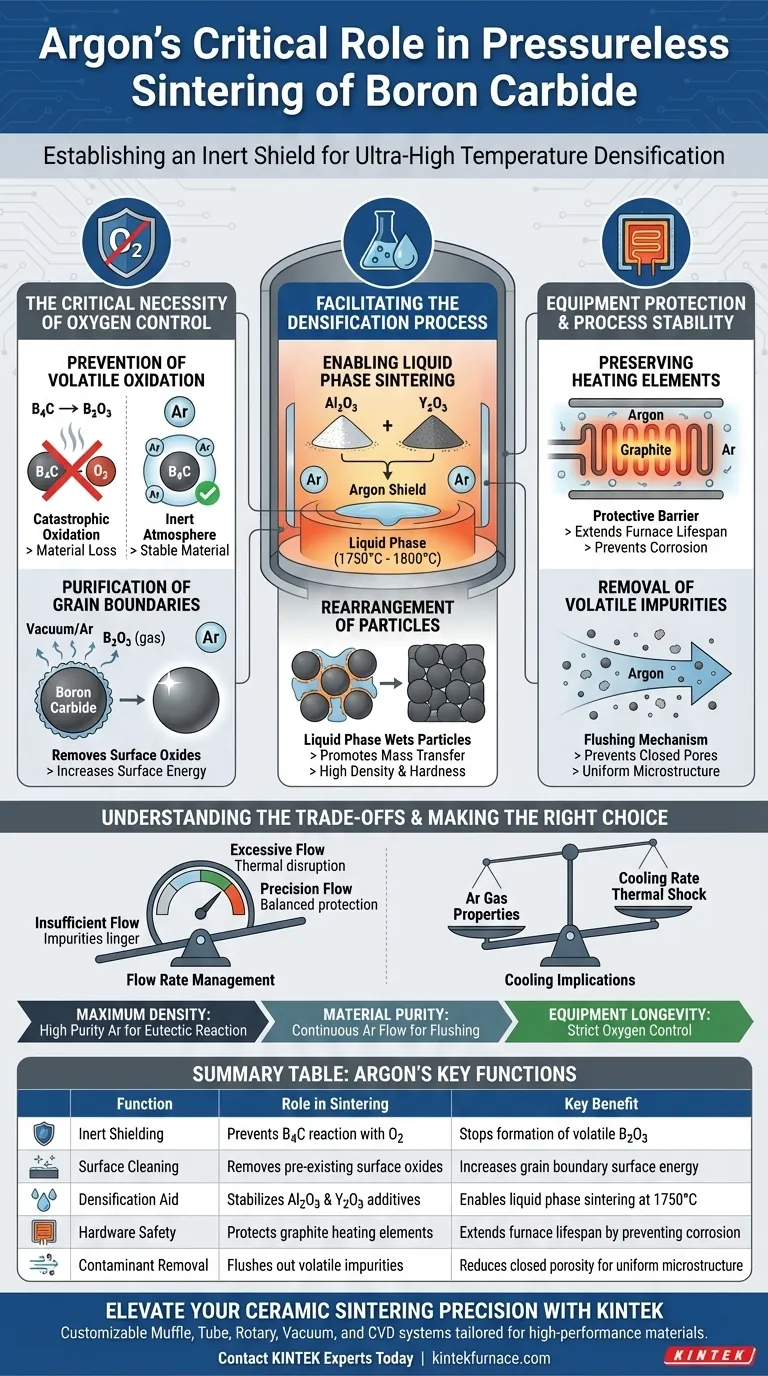
The primary role of an Argon gas environment in pressureless sintering is to establish a chemically inert shield that prevents the catastrophic oxidation of Boron Carbide at ultra-high temperatures. By maintaining an extremely low oxygen partial pressure, Argon prevents Boron Carbide from degrading into volatile Boron Oxide ($B_2O_3$) while simultaneously creating the stable conditions required for sintering aids to densify the ceramic.
Core Takeaway Boron Carbide is notoriously difficult to sinter due to its covalent bonding and susceptibility to oxidation. Argon acts as a critical process enabler: it suppresses the evaporation of material as $B_2O_3$, protects furnace heating elements from corrosion, and ensures sintering aids can successfully form the liquid phase needed to achieve high density.

The Critical Necessity of Oxygen Control
Prevention of Volatile Oxidation
At temperatures exceeding 1800°C, Boron Carbide is highly unstable in the presence of oxygen. Without an inert Argon atmosphere, Boron Carbide reacts to form Boron Oxide ($B_2O_3$).
Unlike stable oxides, $B_2O_3$ is volatile at these temperatures. If it forms, it evaporates, causing significant material loss and preventing the ceramic particles from bonding.
Purification of Grain Boundaries
Argon does more than just prevent new oxidation; it facilitates the removal of existing impurities.
The vacuum or controlled atmosphere promotes the volatilization and removal of pre-existing surface oxide layers on the Boron Carbide particles. This "cleaning" of the grain boundaries increases surface energy, which is a prerequisite for successful densification.
Facilitating the Densification Process
Enabling Liquid Phase Sintering
Pressureless sintering of Boron Carbide often relies on additives, such as Aluminum Oxide ($Al_2O_3$) and Yttrium Oxide ($Y_2O_3$).
The high-purity Argon environment ensures these additives can react chemically without interference. Specifically, it allows them to form a liquid phase at temperatures between 1750°C and 1800°C.
Rearrangement of Particles
Once this liquid phase forms, it wets the solid Boron Carbide particles.
This promotes particle rearrangement and mass transfer. The result is a significantly denser final product achieved at lower temperatures than would be possible without these additives, all while maintaining the material's hardness.
Equipment Protection and Process Stability
Preserving Heating Elements
Sintering furnaces often utilize graphite or other carbon-based heating elements.
These elements are highly susceptible to corrosion and oxidation at operating temperatures. The Argon atmosphere acts as a protective barrier, extending the lifespan of the furnace components by preventing oxygen attack.
Removal of Volatile Impurities
A continuous flow of Argon gas serves as a flushing mechanism.
It carries away volatile impurities and adsorbed gases released during the heating process. By physically sweeping these contaminants out of the hot zone, the Argon flow prevents the formation of closed pores, leading to a more uniform microstructure.
Understanding the Trade-offs
Flow Rate Management
While Argon is protective, the dynamics of gas flow are critical.
Insufficient flow allows volatile impurities to linger near the product, potentially re-depositing or inhibiting densification. Excessive flow can disrupt thermal uniformity or increase consumption costs unnecessarily. Precision flow controllers are essential to balance protection with thermal stability.
Cooling Implications
The choice of gas affects the post-sintering phase.
Inert gas cooling systems often circulate cooled Argon back into the chamber to accelerate production cycles. Because gas density impacts heat dispersal, the specific properties of Argon influence the cooling rate. This must be managed to prevent thermal shock while minimizing cycle times.
Making the Right Choice for Your Goal
To maximize the effectiveness of your sintering process, align your atmospheric controls with your specific material objectives:
- If your primary focus is Maximum Density: Ensure the Argon environment is pure enough to allow the $Al_2O_3$-$Y_2O_3$ system to trigger the eutectic reaction (liquid phase) without oxidation interference.
- If your primary focus is Material Purity: Utilize a continuous, controlled flow of Argon rather than a static atmosphere to actively sweep away volatilized surface oxides and binders.
- If your primary focus is Equipment Longevity: Monitor oxygen partial pressure strictly to prevent the simultaneous degradation of both the Boron Carbide workload and the graphite heating elements.
Success in pressureless sintering relies not just on heat, but on using Argon to create a pristine chemical stage where densification can occur unimpeded.
Summary Table:
| Function | Role in Sintering Process | Key Benefit |
|---|---|---|
| Inert Shielding | Prevents $B_2C$ reaction with oxygen | Stops formation of volatile $B_2O_3$ |
| Surface Cleaning | Removes pre-existing surface oxides | Increases grain boundary surface energy |
| Densification Aid | Stabilizes $Al_2O_3$ and $Y_2O_3$ additives | Enables liquid phase sintering at 1750°C |
| Hardware Safety | Protects graphite heating elements | Extends furnace lifespan by preventing corrosion |
| Contaminant Removal | Flushes out volatile impurities | Reduces closed porosity for uniform microstructure |
Elevate Your Ceramic Sintering Precision with KINTEK
Don't let oxidation compromise your Boron Carbide density or damage your equipment. KINTEK provides industry-leading atmosphere-controlled solutions tailored for high-performance materials. Backed by expert R&D and manufacturing, we offer customizable Muffle, Tube, Rotary, Vacuum, and CVD systems designed to maintain the pristine Argon environments your lab demands.
Ready to optimize your high-temperature process? Contact KINTEK Experts Today to find the perfect furnace for your unique sintering needs.
Visual Guide
References
- J. Q. Wang, Li Hou. Mechanical and Drying Shrinkage Performance Study of Ultra-High-Performance Concrete Prepared from Titanium Slag under Different Curing Conditions. DOI: 10.3390/ma17174201
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why are inert gases like nitrogen and argon used in furnaces? Prevent Oxidation and Ensure Material Purity
- What role does a high-temperature argon atmosphere sintering furnace play in the production of 316L? Master Metallurgy
- How does inert atmosphere heat treating benefit aluminum? Prevent Oxide Buildup for Superior Results
- What is the main purpose of introducing a reducing atmosphere in sintering? Optimize Metal Bonding & Strength
- Why is it necessary to use a high-temperature furnace to pre-fire porous alumina substrates for alloy wettability?
- Why Use a Reducing Atmosphere Furnace for Na4Fe3(PO4)2(P2O7)? Ensure Fe2+ Stability and Battery Performance
- Why use N2 and SF6 protection gas for Mg-Zn-Ca alloy melting? Prevent Combustion and Ensure High Purity
- How is atmosphere control managed during furnace operation? Master Precise Gas Environments for Superior Results



















