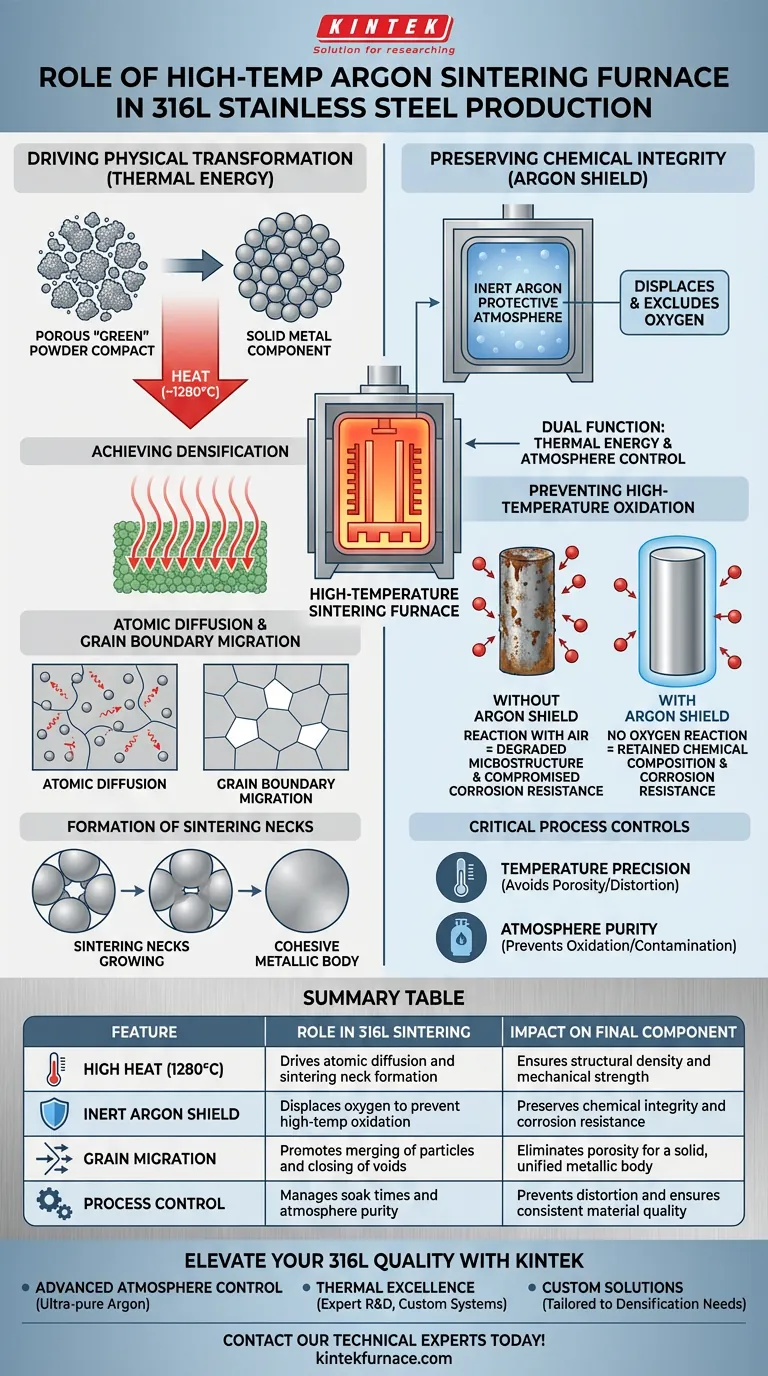
A high-temperature argon atmosphere sintering furnace serves as the critical processing environment that transforms porous 316L stainless steel powder into solid, high-performance metal. It functions by generating extreme heat (typically around 1280°C) to drive atomic densification, while simultaneously enveloping the components in inert argon gas to strictly prevent oxidation during the thermal cycle.
The furnace performs a dual function: the thermal energy drives the physical transformation from "green" powder compact to solid metal through atomic diffusion, while the argon shield preserves the chemical composition to ensure the final product retains the corrosion resistance 316L is known for.
Driving Physical Transformation through Heat
The primary role of the furnace is to provide the energy required to change the physical state of the material without melting it completely.
Achieving Densification
The furnace creates a thermal environment capable of reaching temperatures such as 1280 degrees Celsius.
At this intensity, the metal particles within the porous "green compact" (the pre-sintered shape) begin to bond. This heat is the catalyst for turning a fragile collection of particles into a unified, dense structural component.
Atomic Diffusion and Grain Boundary Migration
The mechanism behind this densification is atomic diffusion. The high heat increases the kinetic energy of the atoms, allowing them to move across particle boundaries.
Simultaneously, the process promotes grain boundary migration. As grains merge and grow, the voids (pores) between particles shrink and eventually close.
Formation of Sintering Necks
As detailed in supplementary technical contexts, this thermal exposure facilitates the formation of sintering necks.
These are the initial connection points between individual steel particles. As the necks grow, the structure solidifies, resulting in a cohesive metallic body.
Preserving Chemical Integrity with Argon
While heat drives the physical structure, the atmosphere controls the chemical quality. Processing 316L stainless steel requires strict protection against reaction with the air.
The Argon Shield
The furnace maintains a high-purity inert argon protective atmosphere.
Argon is a noble gas that does not react with steel. By filling the furnace chamber with argon, the system effectively displaces and excludes atmospheric oxygen.
Preventing High-Temperature Oxidation
Stainless steel is highly susceptible to oxidation when exposed to oxygen at sintering temperatures.
Without the argon shield, the steel would react with oxygen, degrading the material's microstructure. This oxidation would compromise the corrosion resistance of the final part, rendering the 316L alloy ineffective for its intended applications.
Critical Process Controls and Trade-offs
Sintering is a balancing act between thermal kinetics and atmospheric purity. Understanding the potential pitfalls is essential for high-yield production.
Temperature Precision
The temperature must be controlled precisely. If the temperature is too low, atomic diffusion is insufficient, leading to a part that remains porous and mechanically weak.
Conversely, excessive temperatures can lead to distortion or uncontrolled grain growth, which may reduce the mechanical toughness of the component.
Atmosphere Purity
The effectiveness of the process relies entirely on the purity of the argon.
Even trace amounts of oxygen can lead to surface oxidation or internal contamination of the stainless steel matrix. Ensuring a leak-proof environment and high-quality gas flow is non-negotiable for critical components.
Optimizing Production for 316L
To achieve the best results with your sintering furnace, align your process parameters with your specific performance requirements.
- If your primary focus is mechanical strength: Prioritize precise temperature control at the peak (e.g., 1280°C) and adequate soak times to maximize atomic diffusion and sintering neck formation.
- If your primary focus is corrosion resistance: strict management of the argon atmosphere is paramount to prevent even microscopic oxidation of the stainless steel surface.
By strictly controlling both the thermal profile and the inert atmosphere, you ensure the production of 316L components that are both structurally dense and chemically robust.
Summary Table:
| Feature | Role in 316L Sintering | Impact on Final Component |
|---|---|---|
| High Heat (1280°C) | Drives atomic diffusion and sintering neck formation | Ensures structural density and mechanical strength |
| Inert Argon Shield | Displaces oxygen to prevent high-temp oxidation | Preserves chemical integrity and corrosion resistance |
| Grain Migration | Promotes merging of particles and closing of voids | Eliminates porosity for a solid, unified metallic body |
| Process Control | Manages soak times and atmosphere purity | Prevents distortion and ensures consistent material quality |
Elevate Your 316L Component Quality with KINTEK
Precision in sintering is the difference between a fragile part and a high-performance component. At KINTEK, we understand that your 316L stainless steel projects require the perfect balance of thermal kinetics and atmospheric purity.
Why choose KINTEK for your high-temp processing?
- Advanced Atmosphere Control: Our systems ensure ultra-pure argon environments to protect your materials from oxidation.
- Thermal Excellence: Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems designed for extreme temperature precision.
- Custom Solutions: All our lab high-temp furnaces are fully customizable to meet the unique densification needs of your target applications.
Don't compromise on your material's integrity. Contact our technical experts today to find the ideal sintering solution for your lab or production facility!
Visual Guide
References
- Marcelo Broch, María Cristina Moré Farias. Scratch Response of Hollow Cathode Radiofrequency Plasma-Nitrided and Sintered 316L Austenitic Stainless Steel. DOI: 10.3390/coatings14030334
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- How does constant temperature heating equipment affect catalyst precursors? Precision Control for Perovskite Quality
- How are the heating elements arranged in the box type annealing atmosphere furnace? For Uniform Heating and Precise Control
- Why must a high-purity argon protective atmosphere be maintained during mechanical alloying? Ensure Peak Material Purity
- What are the consequences of an improperly controlled furnace atmosphere? Avoid Costly Defects and Safety Hazards
- What materials are used for the furnace structure of the box type annealing atmosphere furnace? Discover Durable, High-Temp Solutions
- What role does a vacuum or atmosphere tube furnace play in the sintering process of Al6061/B4C composites?
- Why is a reducing atmosphere essential for phosphor synthesis? Unlock High-Efficiency Blue Light Activation
- What are the limitations of low vacuum atmosphere furnaces? Understand Trade-offs for Cost-Effective Heat Treatment



















