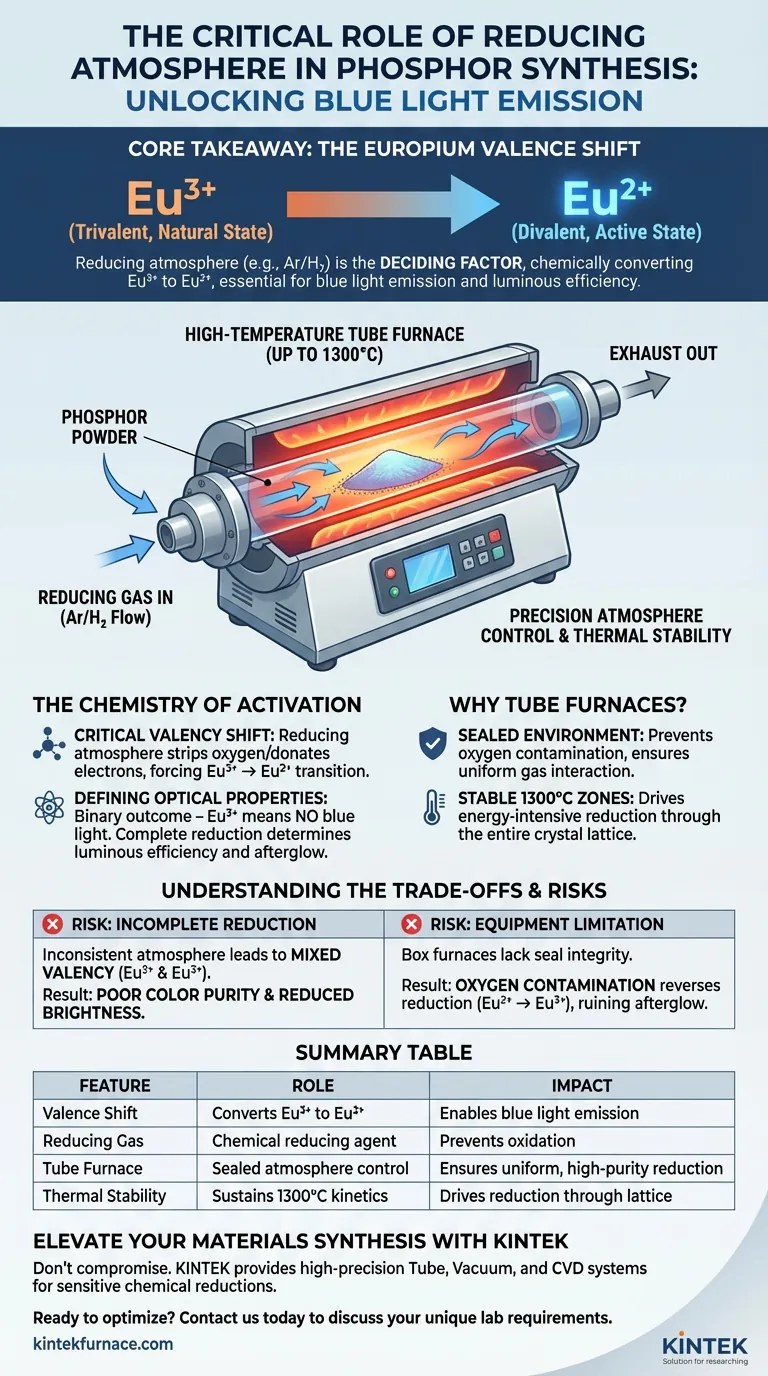
The essential function of a reducing atmosphere during phosphor calcination is to chemically alter the valence state of the dopant element, specifically Europium. While the high temperature (up to 1300°C) facilitates the crystal formation, the reducing gas is the active agent that converts Europium from its natural trivalent state ($Eu^{3+}$) to the required divalent state ($Eu^{2+}$).
Core Takeaway The presence of a reducing atmosphere is the deciding factor in activating blue light emission in phosphors. It drives the chemical reduction of Europium ($Eu^{3+} \rightarrow Eu^{2+}$); without this specific valence shift, the phosphor will fail to exhibit the desired luminous efficiency and afterglow characteristics.
The Chemistry of Activation
The Critical Valency Shift
In the synthesis of specific phosphors, the dopant material—Europium—naturally exists in a trivalent state ($Eu^{3+}$).
However, to function as an effective activator for blue light emission, this element must be chemically reduced to a divalent state ($Eu^{2+}$). The reducing atmosphere provides the necessary chemical environment to strip oxygen or donate electrons, forcing this transition.
Defining Optical Properties
The completion of this reaction is not merely beneficial; it is binary.
If the Europium remains in the $Eu^{3+}$ state, the material will not emit the targeted blue light. The completeness of the reduction directly dictates the final luminous efficiency and the quality of the afterglow.
The Role of the High-Temperature Tube Furnace
Precision Atmosphere Control
A high-temperature tube furnace is uniquely safer and more effective for this process than standard box furnaces.
It creates a sealed, controlled environment where a specific reducing gas (often a mixture like Argon/Hydrogen) can flow consistently over the sample. This ensures that the reducing agent is constantly replenished and interacts uniformly with the phosphor powder.
Thermal Stability for Reaction Kinetics
The reduction of Europium is an energy-intensive reaction requiring temperatures up to 1300°C.
The tube furnace provides stable high-temperature zones that maintain these conditions long enough for the reduction to permeate the entire crystal lattice. This thermal stability ensures that the reduction is not just surface-level but occurs throughout the bulk of the material.
Understanding the Trade-offs
The Risk of Incomplete Reduction
If the reducing atmosphere is inconsistent—due to leaks or improper gas flow rates—you risk creating a mixture of $Eu^{2+}$ and $Eu^{3+}$.
This "mixed valency" results in poor color purity and reduced brightness. Unlike a standard sintering process where structure is the only goal, here the chemical atmosphere is just as critical as the temperature.
Equipment Limitation
While high-temperature box furnaces are excellent for discharging volatiles like $CO_2$ or forming oxide phases in air, they generally lack the seal integrity required for strict reducing atmospheres.
Using the wrong furnace type leads to oxygen contamination, which immediately reverses the reduction process, oxidizing the Europium back to the inactive $Eu^{3+}$ state.
Making the Right Choice for Your Goal
To maximize the quality of your phosphor synthesis, align your process parameters with your specific outcome:
- If your primary focus is Blue Light Emission: Prioritize a verified reducing atmosphere (e.g., Ar/H2) to guarantee the complete conversion of $Eu^{3+}$ to $Eu^{2+}$.
- If your primary focus is Luminous Efficiency: Ensure your tube furnace maintains a stable thermal zone at 1300°C to allow the reduction reaction to reach full completion throughout the batch.
- If your primary focus is Material Purity: Monitor gas flow rates strictly to prevent oxidation, which ruins the afterglow characteristics.
Control the atmosphere as strictly as you control the temperature, because the gas determines the chemistry of the light.
Summary Table:
| Feature | Role in Phosphor Synthesis | Impact on Outcome |
|---|---|---|
| Valence Shift | Converts $Eu^{3+}$ to $Eu^{2+}$ | Enables blue light emission |
| Reducing Gas | Acts as a chemical reducing agent | Prevents oxidation of dopants |
| Tube Furnace | Provides sealed atmosphere control | Ensures uniform, high-purity reduction |
| Thermal Stability | Sustains 1300°C reaction kinetics | Drives reduction through crystal lattice |
Elevate Your Materials Synthesis with KINTEK
Don’t let atmospheric contamination compromise your luminous efficiency. KINTEK provides high-precision Tube, Vacuum, and CVD systems designed specifically for sensitive chemical reductions. Backed by expert R&D and manufacturing, our customizable high-temperature furnaces ensure the strict atmosphere control required for perfect valence shifts in phosphor production.
Ready to optimize your synthesis? Contact us today to discuss your unique lab requirements with our specialists.
Visual Guide
References
- K. A. K. Durga Prasad, D. Haranath. Enhanced blue emission and afterglow properties of Sr2MgSi2O7:Eu2+, Dy3+ phosphors for flexible transparent labels. DOI: 10.1063/5.0230526
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What is a retort furnace and what are its key features? Discover Precision Heating for Superior Material Processing
- How does high-temperature calcination equipment contribute to the conversion of chicken bones into hydroxyapatite?
- What is reducing atmosphere heat treatment? Leverage Precise Chemistry for Pristine Metal Surfaces
- What is the purpose of switching between N2 and H2 in electrical steel annealing? Master Atmosphere Control
- What process environment does a tube atmosphere furnace provide for LMFP? Master Secondary Crystallization
- Why use N2 and SF6 protection gas for Mg-Zn-Ca alloy melting? Prevent Combustion and Ensure High Purity
- How do fixed-bed reactors and heating furnaces ensure accurate reaction data? Master Toluene Degradation Precision
- What types of heat treatment processes require controlled atmospheres? Essential for Surface Protection and Modification



















