Virtually any heat treatment process that requires precise control over a metal's surface properties relies on a controlled atmosphere. This includes common processes such as carburizing, nitriding, and carbonitriding, which add elements to the surface, as well as processes like bright annealing, neutral hardening, and brazing, which must prevent surface reactions like oxidation.
A controlled atmosphere is not merely a passive, protective shield. It is often an active and critical ingredient in the heat treatment recipe, used either to prevent unwanted chemical reactions or to intentionally induce specific changes in the material's surface chemistry.
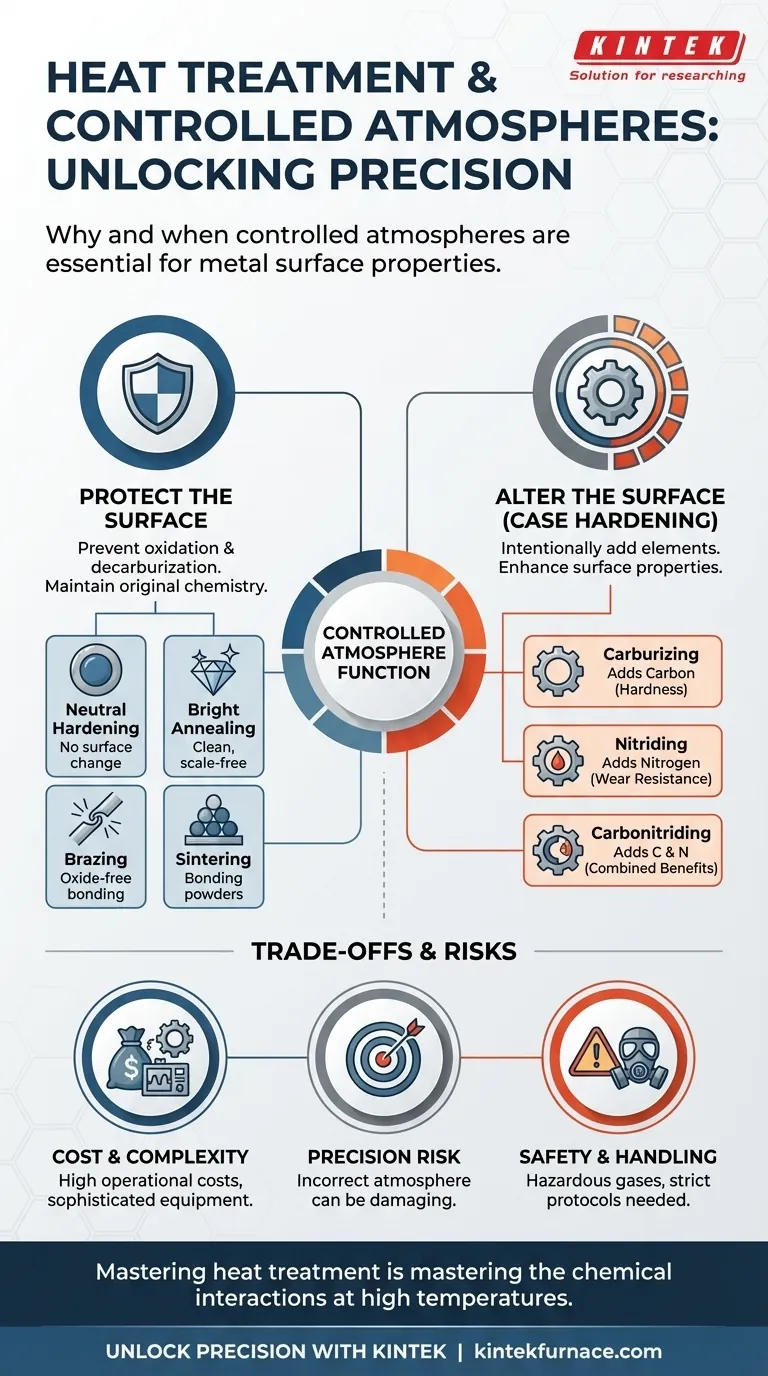
The Two Primary Functions of a Controlled Atmosphere
The decision to use a controlled atmosphere furnace stems from one of two fundamental needs: protecting the existing surface or creating a new one.
To Protect the Material's Surface
The most common reason for a controlled atmosphere is to prevent the hot surface of the metal from reacting with the surrounding air. At high temperatures, steel readily reacts with oxygen (oxidation) and can lose carbon from its surface (decarburization).
A protective or "inert" atmosphere creates a barrier, shielding the part from these unwanted reactions. This ensures the component's surface chemistry and dimensions remain unchanged throughout the thermal cycle.
To Chemically Alter the Material's Surface
The second major function is to intentionally change the surface of the part to enhance its properties. This is known as case hardening.
In these processes, the atmosphere is precisely formulated to act as a carrier, diffusing specific elements like carbon or nitrogen into the steel's surface. This creates a hard, wear-resistant outer "case" while leaving the inner "core" tough and ductile.
Key Processes and Their Atmospheric Needs
Understanding the goal of the process reveals why its atmosphere is so critical.
Surface Modification (Case Hardening)
- Carburizing: This process introduces carbon into the surface of low-carbon steel to increase its hardness. The atmosphere must have a specific, tightly controlled carbon potential to achieve the desired case depth and hardness.
- Nitriding: This process diffuses nitrogen into the steel's surface, forming extremely hard nitride compounds. It often uses an ammonia-based atmosphere, which dissociates at temperature to provide the necessary nitrogen.
- Carbonitriding: As the name implies, this process adds both carbon and nitrogen to the surface, combining benefits of both processes, often at lower temperatures than carburizing.
Surface Protection and Specialized Processes
- Neutral Hardening: The goal is to harden the steel by heating and quenching it without altering its surface chemistry. The atmosphere must be perfectly neutral to the steel's carbon content, preventing both carburization and decarburization.
- Annealing: This process softens metal to improve its ductility. When a clean, scale-free surface is required, it is done in a controlled atmosphere and is often called bright annealing.
- Brazing: This joining process melts a filler metal to bond two components. A controlled atmosphere is essential to prevent the formation of oxides on the base metals, which would inhibit the filler metal from properly wetting and bonding the surfaces.
- Sintering: Used in powder metallurgy, this process heats compacted metal powders to bond them into a solid object. The atmosphere prevents oxidation and can help burn off lubricants used in the compacting stage.
Understanding the Trade-offs and Risks
While essential, controlled atmospheres introduce complexity and potential points of failure.
The Cost of Precision
Implementing and maintaining a controlled atmosphere is a significant operational cost. It involves the expense of industrial gases (nitrogen, hydrogen, argon), sophisticated sensors to monitor gas composition, and furnaces with high integrity to prevent leaks.
The Risk of an Incorrect Atmosphere
An improperly controlled atmosphere can be more damaging than no control at all. For example, an atmosphere intended to be neutral can become carburizing or decarburizing if its carbon potential drifts, ruining the workpiece.
Safety and Handling
Many atmosphere gases are hazardous. Endothermic and nitrogen-methanol atmospheres contain flammable hydrogen and toxic carbon monoxide. Ammonia used for nitriding is also toxic and corrosive. Safe storage, handling, and ventilation are non-negotiable requirements.
Making the Right Choice for Your Goal
The specific heat treatment process and its atmosphere are chosen based on the desired final properties of the component.
- If your primary focus is maximum surface hardness and wear resistance: A case hardening process like nitriding or carburizing is the required path.
- If your primary focus is hardening a component without changing its surface: Neutral hardening in a precisely balanced protective atmosphere is the correct choice.
- If your primary focus is producing a clean, oxide-free surface after softening or joining: Bright annealing or brazing in an inert or reducing atmosphere is necessary.
Ultimately, mastering heat treatment is mastering the chemical interactions between the metal and its surrounding atmosphere at high temperatures.

Summary Table:
| Process Type | Key Processes | Atmospheric Function |
|---|---|---|
| Surface Modification | Carburizing, Nitriding, Carbonitriding | Adds elements (e.g., carbon, nitrogen) to alter surface properties |
| Surface Protection | Neutral Hardening, Bright Annealing, Brazing, Sintering | Prevents oxidation and decarburization for clean, unchanged surfaces |
Unlock Precision in Your Heat Treatment Processes with KINTEK
Are you struggling with surface oxidation, inconsistent hardness, or material degradation during heat treatment? KINTEK specializes in advanced high-temperature furnace solutions tailored to your exact needs. Leveraging exceptional R&D and in-house manufacturing, we offer a diverse product line including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we can precisely meet your unique experimental requirements, whether you're involved in case hardening, annealing, brazing, or sintering.
Don't let atmospheric challenges hold back your innovations—contact us today to discuss how our solutions can enhance your lab's efficiency, improve product quality, and reduce operational risks. Let's achieve superior results together!
Visual Guide
Related Products
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- What is the main purpose of heat treatment? Transform Metal Properties for Superior Performance
- What industries commonly use inert atmosphere heat treating? Key Applications in Military, Automotive, and More
- What are the two main types of atmosphere furnaces and their characteristics? Choose the Right Furnace for Your Lab
- What is the use of nitrogen in furnace? Prevent Oxidation for Superior Heat Treatment
- Why is moisture control critical in inert atmosphere heat treating? Prevent Oxidation and Ensure Material Integrity



















