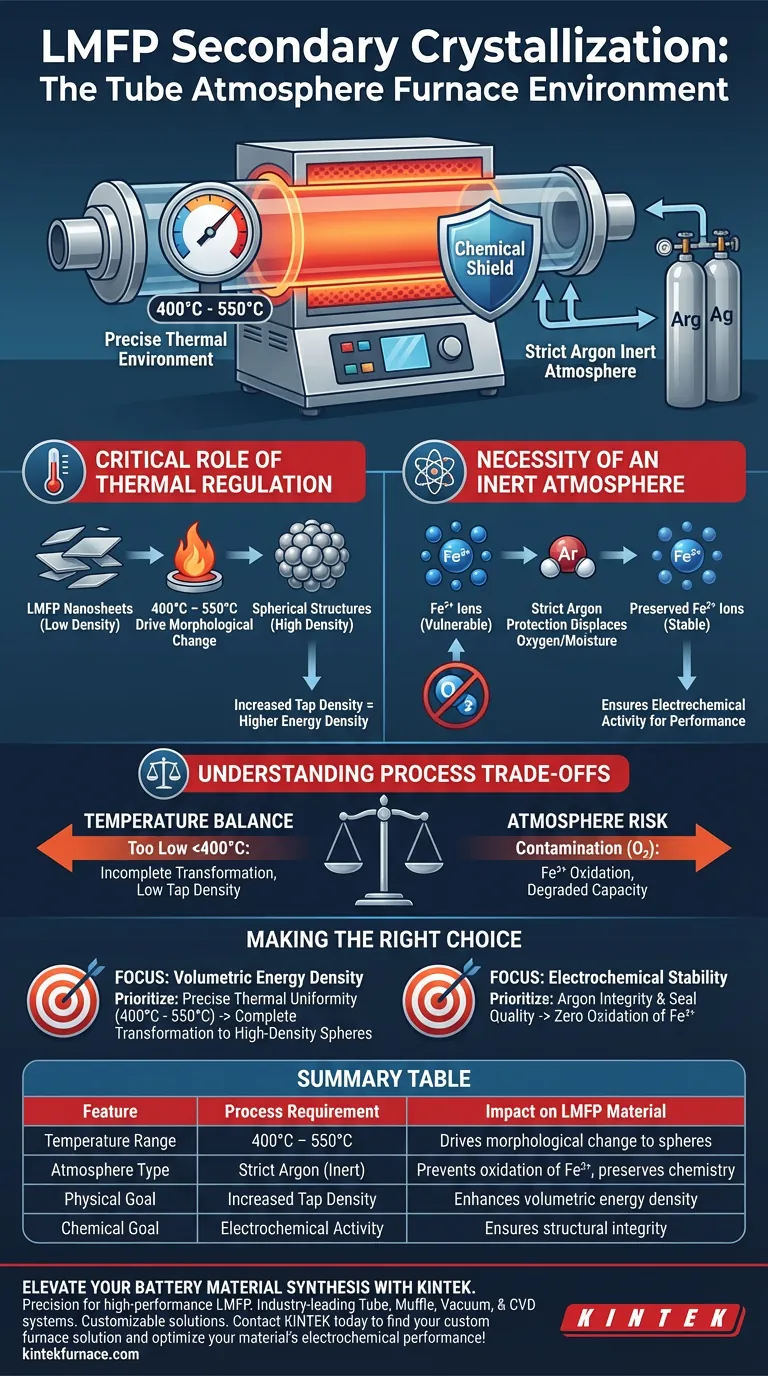
During secondary crystallization, a tube atmosphere furnace creates a precise thermal environment between 400°C and 550°C encapsulated within a strict argon inert atmosphere. This controlled setting is critical for transforming the material's physical structure while simultaneously protecting its chemical composition from environmental degradation.
The furnace environment serves a dual purpose: it provides the thermal energy necessary to reshape nanosheets into dense spheres for higher energy density, while the inert atmosphere acts as a chemical shield to prevent the oxidation of iron, preserving the material's electrochemical performance.

The Critical Role of Thermal Regulation
Precise Temperature Windows
The tube atmosphere furnace maintains a specific temperature range of 400°C to 550°C for this process step.
Driving Morphological Change
This thermal energy is not arbitrary; it is the catalyst for a major structural transformation. It induces the LMFP nanosheet structures to evolve into spherical shapes.
Increasing Material Density
The shift from sheets to spheres is essential for practical application. This morphological change significantly increases the tap density of the material, which directly correlates to how much energy can be packed into a battery cell.
The Necessity of an Inert Atmosphere
Strict Argon Protection
The furnace operates under a strictly controlled argon atmosphere. This displaces oxygen and moisture, creating a chemically neutral environment for the reaction.
Preserving Chemical Integrity
The primary function of this inert gas is to prevent the oxidation of transition metal ions, specifically Iron (II) (Fe2+).
Ensuring Electrochemical Activity
If Fe2+ were allowed to oxidize at these high temperatures, the material would lose its structural integrity. By maintaining a pure argon environment, the furnace ensures the final LMFP material retains the electrochemical activity required for high-performance batteries.
Understanding the Process Trade-offs
The Balance of Temperature
Maintaining the temperature strictly between 400°C and 550°C is vital. If the temperature is too low, the morphological transformation from nanosheets to spheres may remain incomplete, resulting in low tap density.
The Risk of Atmosphere Contamination
The process relies entirely on the purity of the argon environment. Any failure in the furnace's sealing or gas flow can introduce oxygen. Even trace amounts of oxidation can compromise the Fe2+ ions, rendering the precise thermal treatment useless by degrading the material's final electrochemical capacity.
Making the Right Choice for Your Goal
To optimize your LMFP production, align your furnace parameters with your specific material targets:
- If your primary focus is Volumetric Energy Density: Prioritize precise thermal uniformity within the 400°C–550°C range to ensure the complete transformation of nanosheets into high-density spheres.
- If your primary focus is Electrochemical Stability: Focus on the integrity of the argon flow and seal quality to guarantee zero oxidation of the sensitive Fe2+ ions during the heating cycle.
Success in secondary crystallization relies on the rigorous synchronization of thermal precision and atmospheric purity.
Summary Table:
| Feature | Process Requirement | Impact on LMFP Material |
|---|---|---|
| Temperature Range | 400°C – 550°C | Drives morphological change from nanosheets to spheres |
| Atmosphere Type | Strict Argon (Inert) | Prevents oxidation of Fe2+ ions and preserves chemistry |
| Physical Goal | Increased Tap Density | Enhances volumetric energy density for battery cells |
| Chemical Goal | Electrochemical Activity | Ensures structural integrity for high-performance use |
Elevate Your Battery Material Synthesis with KINTEK
Precision is the difference between a failed batch and high-performance LMFP. At KINTEK, we provide industry-leading Tube, Muffle, Vacuum, and CVD systems specifically engineered to maintain the rigorous thermal uniformity and atmospheric purity required for secondary crystallization.
Backed by expert R&D and world-class manufacturing, our lab high-temp furnaces are fully customizable to meet your unique research or production needs. Don't let oxygen contamination or temperature fluctuations compromise your energy density.
Contact KINTEK today to find your custom furnace solution and optimize your material's electrochemical performance!
Visual Guide
References
- Shaojun Liu, Chengguo Sun. Freeze-Drying-Assisted Preparation of High-Compaction-Density LiMn0.69Co0.01Fe0.3PO4 Cathode Materials with High-Capacity and Long Life-Cycle for Lithium Ion Batteries. DOI: 10.3390/batteries10040114
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
People Also Ask
- What is the core difference between box and atmosphere furnaces? Choose the Right Equipment for Your Lab
- Which factors influence the radial equivalent thermal conductivity of steel coils? Key Impacts on Annealing Efficiency
- What are the considerations for air atmosphere and cooling in Inconel 625 heat treatment? Optimize 3D Part Stability
- Why are continuous controlled atmosphere furnaces critical for MIM steel parts? Achieve High-Density Sintering
- Why is a rotameter essential for controlling the atmosphere within an oily sludge pyrolysis reactor? Master Gas Flow Control
- Why is stress relief annealing essential for SLM titanium scaffolds? Ensure Durability and Fatigue Resistance
- What is the primary purpose of using a small controlled electric furnace? Optimize Black Liquor Pyrolysis for Research
- How does a high-temperature pyrolysis furnace convert EFB fibers to biochar? Master Precise Thermal Carbonization



















