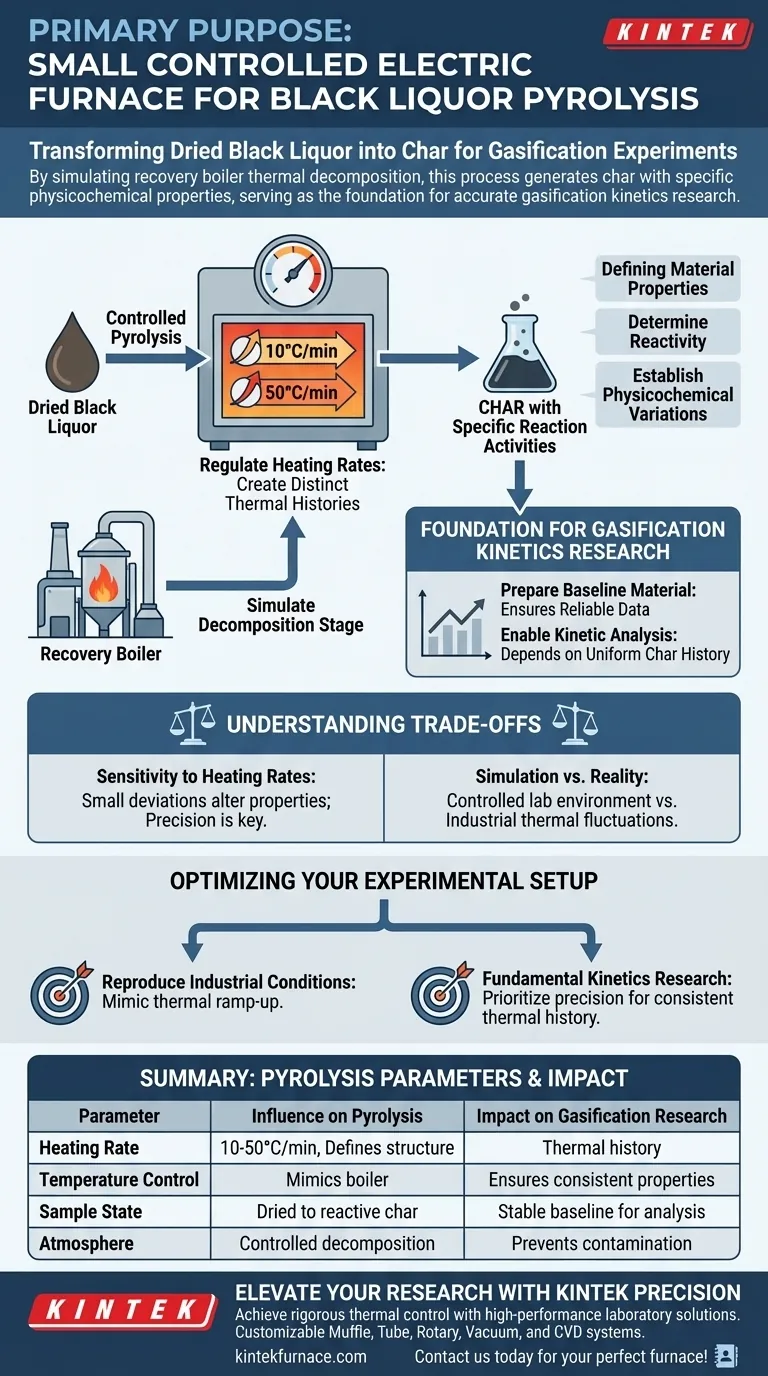
The primary purpose of using a small controlled electric furnace is to transform dried black liquor into char with precisely defined reaction activities. By rigorously controlling the heating environment and applying specific heating rates—such as 10°C/min or 50°C/min—researchers can create samples with distinct thermal histories essential for downstream testing.
By simulating the thermal decomposition stage of a recovery boiler, this process generates char with specific physicochemical properties, serving as the necessary foundation for accurate gasification kinetics research.

Precise Control of Thermal History
Regulating Heating Rates
The electric furnace allows for the exact application of heating rates, specifically varying between slower rates like 10°C/min and faster rates like 50°C/min.
This control is critical because the rate at which black liquor is heated directly alters the resulting material structure.
Simulating Decomposition
The pyrolysis process within the furnace is designed to mimic the thermal decomposition stage that occurs inside a full-scale recovery boiler.
This simulation provides a controlled laboratory environment to observe how black liquor behaves under thermal stress before it undergoes gasification.
Defining Material Properties for Research
Creating Specific Reaction Activities
The ultimate goal of using the furnace is not just to burn the liquor, but to produce char with specific reaction activities.
By manipulating the heating profile, you determine the reactivity of the final char sample.
Establishing Physicochemical Variations
Different thermal histories result in char samples with unique physicochemical properties.
These variations are intentional, allowing researchers to study how different decomposition conditions affect the fuel's quality.
Foundation for Gasification Kinetics
Preparing the Baseline Material
The char produced in this furnace serves as the "foundational material" for subsequent experiments.
Without this controlled pyrolysis step, the starting material for gasification would be inconsistent, leading to unreliable data.
Enabling Kinetic Analysis
Accurate gasification kinetics research depends entirely on the uniformity and known history of the char being tested.
The furnace ensures that the char entering the gasification phase has a documented and controlled thermal past.
Understanding the Trade-offs
Sensitivity to Heating Rates
It is important to recognize that even small deviations in the heating rate can significantly alter the char's physicochemical properties.
If the furnace control is imprecise, the "specific reaction activity" will drift, potentially invalidating the comparison between samples.
Simulation vs. Reality
While the furnace simulates a recovery boiler, it remains a small-scale, controlled environment.
The precise 10°C/min or 50°C/min rates are experimental constants that may not perfectly capture the chaotic thermal fluctuations of an industrial boiler.
Optimizing Your Experimental Setup
To ensure your black liquor pyrolysis yields useful data for gasification, consider your specific research goals:
- If your primary focus is reproducing industrial conditions: Select heating rates that most closely mimic the thermal ramp-up of the target recovery boiler.
- If your primary focus is fundamental kinetics research: Prioritize the precision of the electric furnace to maintain a consistent thermal history across all samples.
The quality of your gasification data is directly dependent on the precision with which you control the initial pyrolysis in the electric furnace.
Summary Table:
| Parameter | Influence on Pyrolysis | Impact on Gasification Research |
|---|---|---|
| Heating Rate | 10°C/min to 50°C/min | Defines material structure and thermal history |
| Temperature Control | Mimics recovery boiler stages | Ensures consistent physicochemical properties |
| Sample State | Dried liquor to reactive char | Provides a stable baseline for kinetic analysis |
| Atmosphere | Controlled decomposition | Prevents unintended oxidation or contamination |
Elevate Your Research with KINTEK Precision
Achieve the rigorous thermal control necessary for accurate gasification kinetics with KINTEK’s high-performance laboratory solutions. Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique experimental heating rates and atmosphere requirements.
Whether you are simulating industrial recovery boilers or conducting fundamental materials research, KINTEK provides the reliability your data demands. Contact us today to find the perfect furnace for your lab!
Visual Guide
References
- F. Bueno, José Luis Sánchez. CO₂ Gasification of Black Liquor Char under isothermal and dynamic conditions. DOI: 10.26754/jji-i3a.202512008
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
People Also Ask
- What are the maintenance points for the box type annealing atmosphere furnace? Ensure Consistent Performance and Safety
- What protective role does a constant flow of inert gas play in dynamic atmosphere sintering? Enhance Material Integrity
- Why compare air and nitrogen atmospheres in CZTS post-annealing? Isolate Oxygen's Impact for Higher Efficiency
- What are the characteristics and uses of hydrogen atmospheres in furnaces? Unlock Clean Metal Processing
- Why is a nitrogen atmosphere necessary when calcining modified graphite felt? Prevent Burnout & Ensure Purity
- What is an atmosphere protection muffle furnace? Unlock Precise Heat Treatment in Controlled Environments
- What is gas quenching in steel part treatment? Achieve Superior Hardness with Controlled Cooling
- What is inert atmosphere heat treating? Prevent Oxidation for Superior Material Quality



















