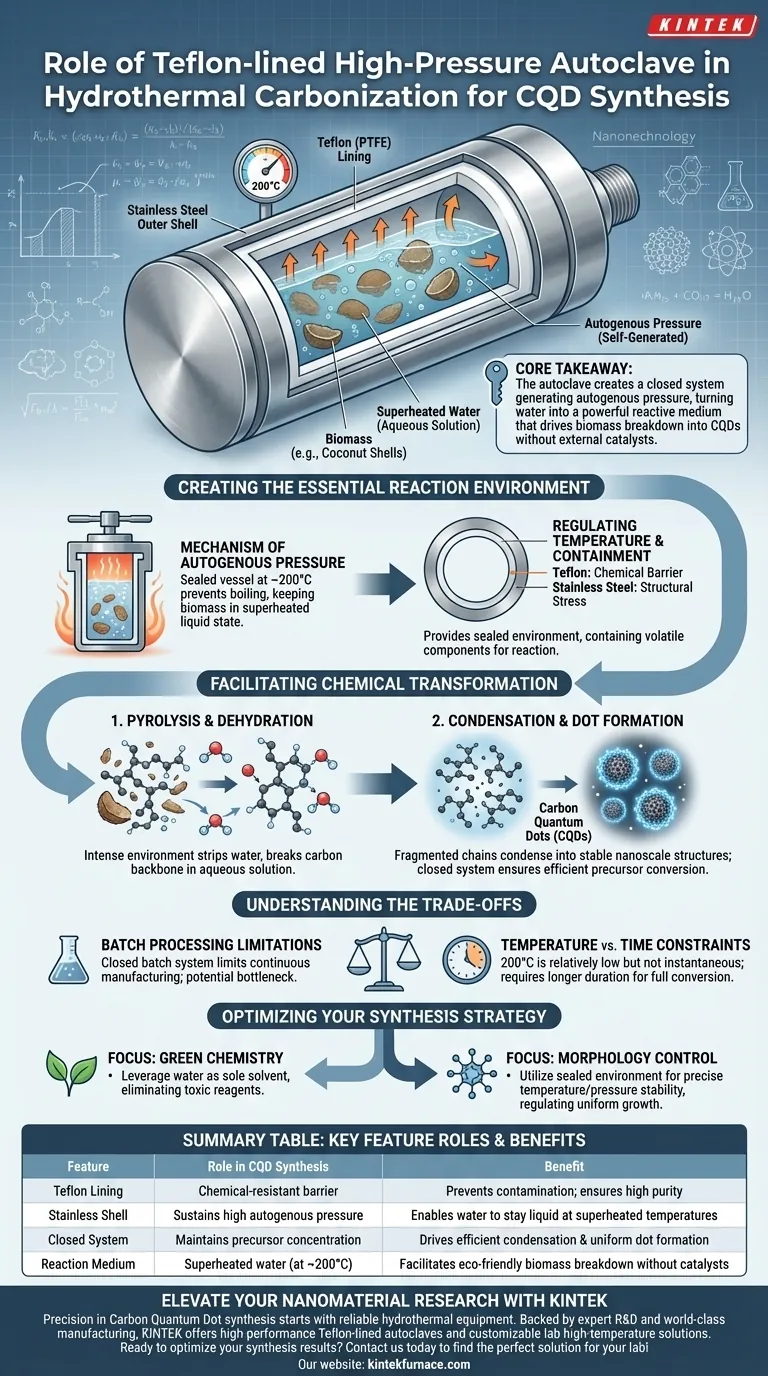
The Teflon-lined high-pressure autoclave acts as the fundamental containment vessel that enables the hydrothermal carbonization of biomass. By sealing the reaction mixture, it allows you to subject aqueous solutions to high temperatures (typically 200°C) and high pressures, facilitating the breakdown of raw materials into stable Carbon Quantum Dots (CQDs) without external chemical catalysts.
Core Takeaway The autoclave’s primary function is to create a closed system that generates autogenous pressure. This pressure alters the properties of water, turning it into a powerful reactive medium that drives the pyrolysis, dehydration, and condensation of biomass into nanostructures at relatively low temperatures.

Creating the Essential Reaction Environment
The Mechanism of Autogenous Pressure
The defining feature of this process is that the pressure is autogenous, meaning it is self-generated.
When the sealed autoclave is heated to roughly 200°C, the water inside creates its own pressure as it attempts to expand against the rigid container.
This pressurized environment prevents the water from boiling away, keeping the biomass submerged in a superheated liquid state that is highly reactive.
Regulating Temperature and Containment
The Teflon lining serves as a chemical barrier, while the stainless steel outer shell handles the structural stress.
This combination provides a sealed environment capable of sustaining high temperatures safely.
It ensures that volatile components formed during the breakdown of biomass—such as coconut shells—are contained and forced to react, rather than escaping as gas.
Facilitating Chemical Transformation
Driving Pyrolysis and Dehydration
Inside this high-pressure cooker, complex biomass structures undergo pyrolysis and dehydration.
The intense environment strips water molecules from the organic chains and breaks down the carbon backbone of the raw material.
This occurs efficiently in the aqueous solution, a stark contrast to dry pyrolysis which often requires significantly higher energy inputs.
Condensation and Dot Formation
Following decomposition, the system promotes condensation.
The fragmented carbon chains reassemble and condense into stable, nanoscale structures known as Carbon Quantum Dots.
Because the system is closed, the concentration of precursors remains consistent, allowing for the efficient conversion of raw material into the desired nanomaterials.
Understanding the Trade-offs
Batch Processing Limitations
The autoclave process operates as a closed batch system.
While this ensures a controlled environment for reaction consistency, it limits your ability to perform continuous manufacturing, potentially creating a bottleneck for large-scale production.
Temperature vs. Time Constraints
While 200°C is considered a "relatively low temperature" compared to other synthesis methods, it is not instantaneous.
Hydrothermal carbonization is a time-dependent process; the "gentler" heat requires a longer duration to fully convert biomass compared to flash pyrolysis methods.
Optimizing Your Synthesis Strategy
To get the most out of your hydrothermal carbonization process, align your equipment use with your specific research goals.
- If your primary focus is Green Chemistry: Leverage the autoclave's ability to use water as the sole solvent, eliminating the need for toxic chemical reagents or harsh acids.
- If your primary focus is Morphology Control: Utilize the sealed environment to maintain precise temperature and pressure stability, which regulates the condensation rate and uniform growth of the quantum dots.
The Teflon-lined autoclave is ultimately an instrument of controlled chaos, forcing organic matter to reorganize into highly valuable nanostructures through heat and pressure alone.
Summary Table:
| Feature | Role in CQD Synthesis | Benefit |
|---|---|---|
| Teflon Lining | Provides a chemical-resistant barrier | Prevents contamination; ensures high purity |
| Stainless Shell | Sustains high autogenous pressure | Enables water to stay liquid at superheated temperatures |
| Closed System | Maintains precursor concentration | Drives efficient condensation and uniform dot formation |
| Reaction Medium | Superheated water (at ~200°C) | Facilitates eco-friendly biomass breakdown without catalysts |
Elevate Your Nanomaterial Research with KINTEK
Precision in Carbon Quantum Dot synthesis starts with reliable hydrothermal equipment. Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with high-performance Teflon-lined autoclaves designed to withstand the rigors of hydrothermal carbonization.
Whether you are processing biomass at 200°C or developing advanced nanostructures, our customizable lab high-temperature solutions provide the stability and safety your research demands.
Ready to optimize your synthesis results? Contact us today to find the perfect customizable solution for your lab!
Visual Guide
References
- A. C. W. W. M. N. Peshala Koswatta, Atula S. D. Sandanayaka. Boosting Solar Cell Efficiency: Enhancing Dye-Sensitized Solar Cell Performance with Carbon Quantum Dots and Titanium Dioxide Nanostructures from Sri Lankan Ilmenite. DOI: 10.1021/acsomega.5c02272
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering and Brazing Furnace
People Also Ask
- What are the thermal properties of alumina tubes? Discover Their High-Temp Durability and Stability
- What is the function of a laboratory drying oven in SnO2 film pre-treatment? Ensure Crack-Free Film Stabilization
- What is the water-saving benefit of using a water circulating vacuum pump? Save Over 10 Tons of Water Daily
- What is the range of internal volumes for Laboratory Type Furnaces? Choose the Right Size for Your Lab Needs
- What is the primary function of a high-energy planetary ball mill? Unlock Nanoscale Ceramic Pretreatment
- What is the specific utility of crucibles in high-temperature lab applications? Precision & Thermal Integrity
- Why is a laboratory vacuum drying oven utilized for recovered carbon black? Preserve rCB Integrity and Pore Structure
- What function does a planetary ball mill perform in LiFePO4/C synthesis? Optimize Battery Material Conductivity



















