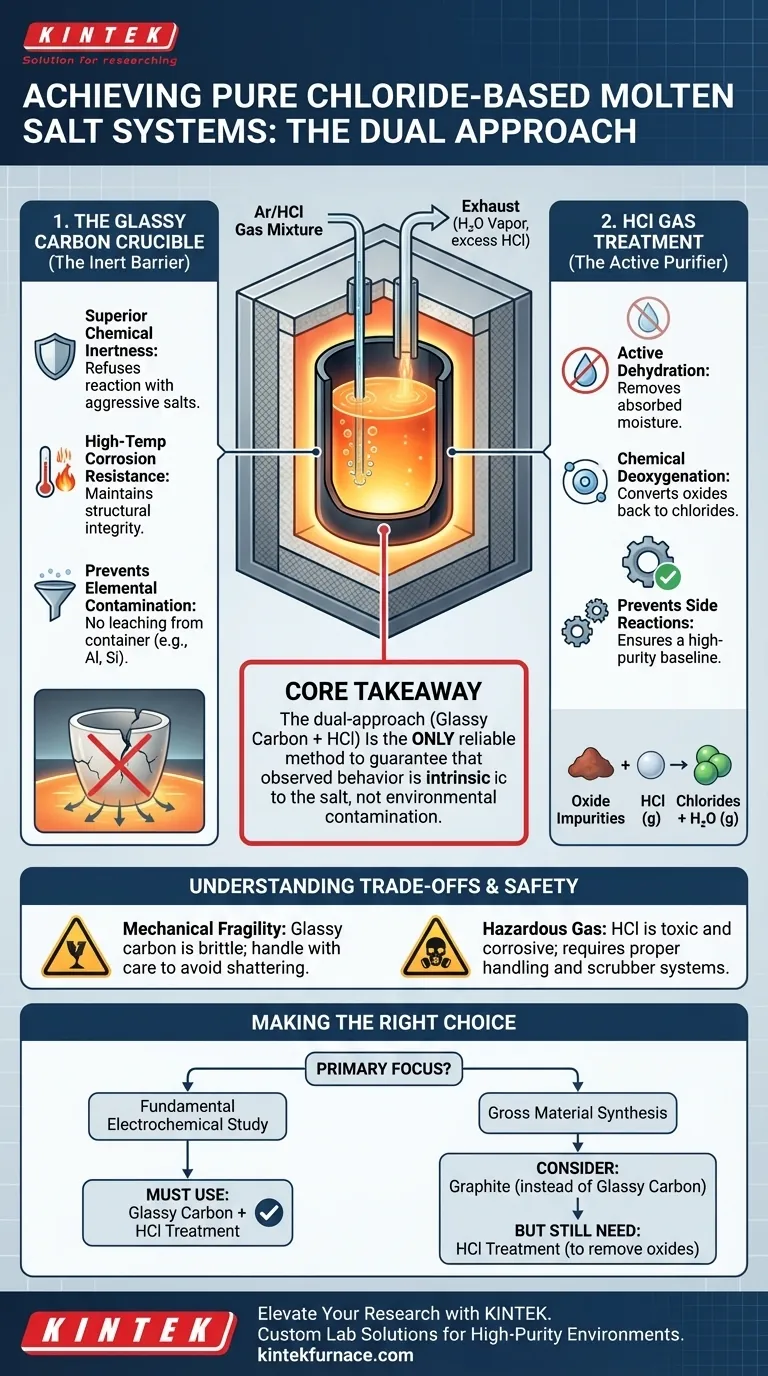
The combination of a glassy carbon crucible and HCl gas treatment is the standard protocol for establishing a chemically pure, stable molten salt environment.
Specifically, the glassy carbon crucible acts as a strictly inert physical barrier that prevents the corrosive molten salt from dissolving its container. Simultaneously, the HCl gas treatment performs an active chemical purification, stripping away microscopic impurities like moisture and oxygen that would otherwise cause unwanted side reactions.
Core Takeaway Chloride-based molten salts are highly susceptible to contamination from both their container and the surrounding atmosphere. Utilizing glassy carbon ensures the vessel itself does not degrade into the melt, while HCl gas actively reverses oxidation and hydrolysis, ensuring the electrolyte remains chemically defined and stable for sensitive electrochemical studies.

The Role of the Glassy Carbon Crucible
Superior Chemical Inertness
Standard ceramic crucibles, such as alumina or porcelain, often fail when exposed to aggressive chloride melts.
The salts can attack the ceramic binder or the material itself, leaching impurities into the melt. Glassy carbon is chemically inert, meaning it refuses to react with the molten salt even at high temperatures.
Resistance to High-Temperature Corrosion
Molten salts are highly corrosive environments that accelerate the degradation of most materials.
Glassy carbon offers exceptional thermal stability and corrosion resistance. It maintains its structural integrity without flaking or dissolving, ensuring the physical container does not become a variable in your experiment.
Prevention of Elemental Contamination
The primary goal of using glassy carbon is to maintain the purity of the electrolyte system.
By resisting corrosion, the crucible ensures that no foreign elements (like aluminum or silicon from ceramics) migrate into the salt. This is critical for studying phenomena like dendrite growth, where even trace impurities can alter results.
The Function of HCl Gas Treatment
Active Dehydration
Chloride salts are naturally hygroscopic, meaning they absorb moisture from the air even before they are melted.
Simply heating the salt is often insufficient to remove all water. Passing an Argon/HCl gas mixture through the melt drives off residual moisture that physical heating alone cannot remove.
Chemical Deoxygenation
Oxygen is a pervasive impurity in chloride systems that can lead to the formation of insoluble oxides or oxychlorides.
The HCl gas triggers a chemical reaction that converts these oxide impurities back into chlorides, releasing water vapor as a byproduct. This effectively "scrubs" oxygen out of the system.
Prevention of Side Reactions
If moisture and oxygen remain in the melt, they participate in parasitic electrochemical reactions.
These side reactions can obscure the behavior of the material you are trying to study. By utilizing a 2-hour HCl treatment, you eliminate the reactants that cause these interferences, ensuring a high-purity baseline.
Understanding the Trade-offs
Mechanical Fragility
While glassy carbon is chemically robust, it is mechanically brittle.
It does not withstand thermal shock or physical impact well. Rapid heating or cooling, or dropping the crucible, can result in catastrophic failure/shattering.
Handling Hazardous Gas
The use of HCl gas introduces significant safety and equipment requirements.
HCl is corrosive to metal furnace components and toxic to humans. You must ensure your experimental setup includes proper gas handling, corrosion-resistant tubing, and an effective exhaust scrubber system.
Making the Right Choice for Your Goal
To ensure your experimental setup aligns with your objectives, apply these guidelines:
- If your primary focus is fundamental electrochemical study: You must use both glassy carbon and HCl treatment; even trace oxides will alter redox potentials and dendrite formation.
- If your primary focus is gross material synthesis: You may potentially substitute glassy carbon for denser graphite, but omitting the HCl step will likely result in a product contaminated with oxides.
Ultimately, this dual-approach is the only reliable method to guarantee that the behavior you observe is intrinsic to the salt, rather than a result of environmental contamination.
Summary Table:
| Component | Primary Function | Key Benefit |
|---|---|---|
| Glassy Carbon Crucible | Chemical Inertness | Prevents container leaching and elemental contamination (Al, Si). |
| HCl Gas Treatment | Active Purification | Removes moisture (dehydration) and converts oxides back to chlorides. |
| Argon Carrier Gas | Atmosphere Control | Provides a stable environment for the HCl treatment process. |
| High-Temp Stability | Corrosion Resistance | Maintains structural integrity in aggressive chloride environments. |
Elevate Your Molten Salt Research with KINTEK
Precision in electrochemical studies demands a contaminant-free environment. KINTEK provides the specialized lab equipment necessary to maintain the highest purity standards. Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable high-temperature furnaces designed for sensitive salt treatments.
Whether you are studying dendrite growth or advanced material synthesis, our technical team is ready to help you configure the perfect setup for your unique needs.
Contact us today to find your custom lab solution
Visual Guide
References
- Kui Liu, Wei‐Qun Shi. Operando Characterization of Uranium Dendrite Growth in High‐Temperature Molten Salt Electrochemistry by Synchrotron X‐Ray Tomography and Diffraction. DOI: 10.1002/advs.202502345
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is the function of high-vacuum encapsulated quartz tubes for Ce2(Fe, Co)17? Ensure Phase Purity and Stability
- What are the technical requirements for the Quartz Boat used as a precursor container in the CVD growth of 2D In2Se3?
- Why is the use of high-purity alumina crucibles essential for the synthesis of Ni3In2Se2? | Precision Material Purity
- How does a sealed high-purity graphite reaction box function? Optimize Sb-Ge Thin Film Selenization
- Why is an external cooling system vital for high-temperature furnace stability? Protect Your Research Integrity
- What factors affect the light transmittance of alumina tubes? Balance Clarity and Durability for Your Lab
- What is the function of a vacuum rotary vane pump in hydrogen measurement? Ensure High-Purity Gas Analysis Baseline
- How does a nitrogen nozzle system influence the quality of components? Optimize Cooling for Structural Integrity



















