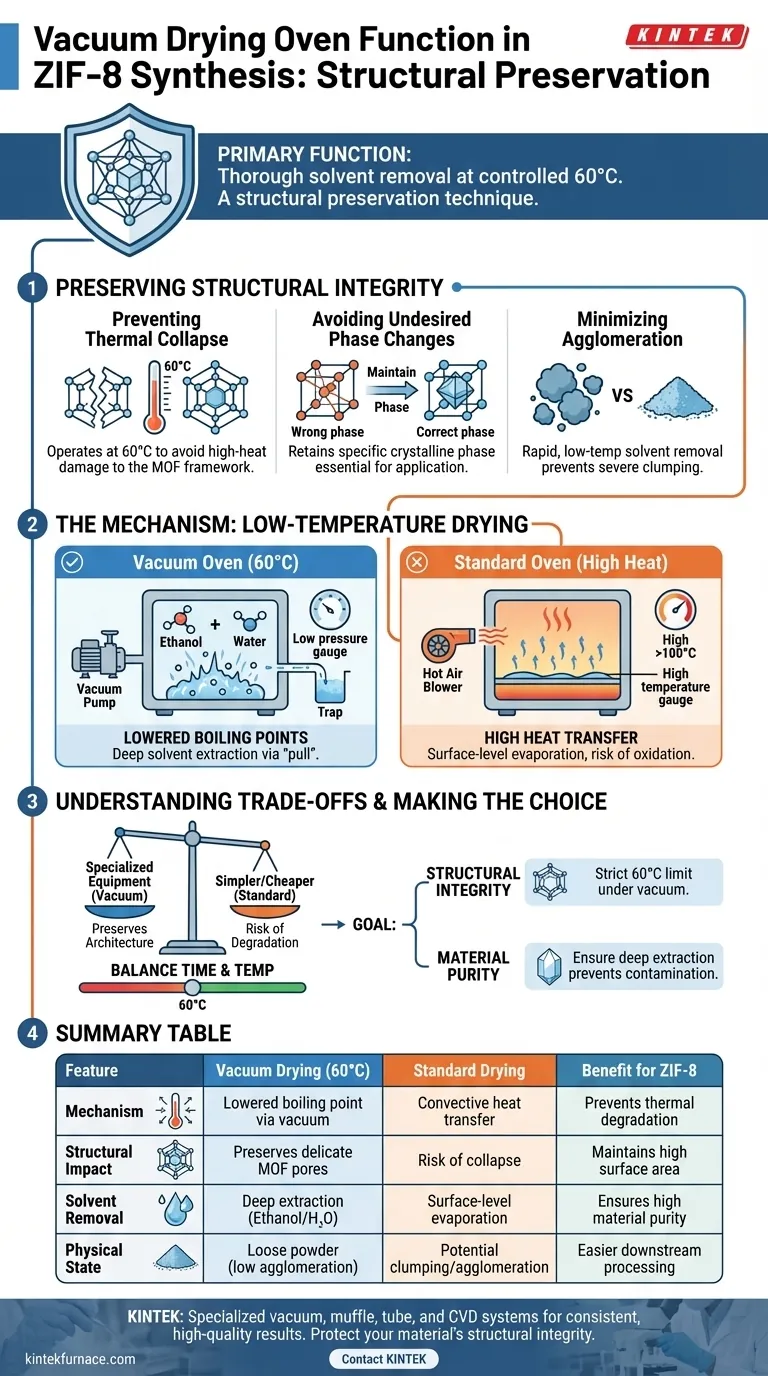
In the synthesis of ZIF-8 precursors, the primary function of a vacuum drying oven is to thoroughly remove residual ethanol and moisture at a controlled temperature of 60 degrees Celsius. By reducing the pressure within the chamber, the oven lowers the boiling point of these solvents, allowing them to evaporate efficiently without requiring high heat.
Core Takeaway: The vacuum drying process is not just about removing liquid; it is a structural preservation technique. By enabling drying at lower temperatures, it prevents the thermal collapse of the metal-organic framework (MOF) and avoids undesired phase changes, ensuring the delicate pore structure remains intact.

Preserving Structural Integrity
Preventing Thermal Collapse
ZIF-8 precursors are sensitive to high temperatures.
Subjecting the material to the high heat required for drying in a standard oven can lead to the collapse of the metal-organic framework (MOF).
The vacuum oven mitigates this risk by operating effectively at a moderate 60 degrees Celsius.
Avoiding Undesired Phase Changes
Beyond simple structural collapse, excessive heat can alter the chemical phase of the precursor.
Maintaining a lower drying temperature ensures the material retains the specific crystalline phase necessary for its final application.
This precision preserves the fundamental properties of the ZIF-8 material.
Minimizing Agglomeration
Drying under vacuum conditions helps maintain the precursor as a loose powder.
Rapid and low-temperature solvent removal prevents the material from undergoing severe agglomeration, or clumping, which can occur during slower or hotter drying processes.
This ensures the final powder retains a high surface area and is easy to handle.
The Mechanism of Low-Temperature Drying
Lowering Solvent Boiling Points
The core advantage of the vacuum oven is physical, not chemical.
By creating a vacuum environment, the atmospheric pressure surrounding the solvents is significantly reduced.
This depression allows ethanol and moisture to boil off at temperatures far below their standard boiling points.
Thorough Solvent Removal
Removing solvents trapped within the porous structure of a MOF is challenging.
The vacuum environment exerts a "pull" that extracts deep-seated ethanol and moisture more effectively than heat alone.
This ensures the precursor is completely dry, which is critical for the accuracy of subsequent processing steps.
Understanding the Trade-offs
Vacuum Drying vs. Standard Drying
While a standard drying oven is simpler and cheaper, it is unsuitable for ZIF-8 synthesis.
Standard ovens rely on heat transfer through air, which requires higher temperatures to achieve the same level of dryness, significantly increasing the risk of oxidation and structural degradation.
The trade-off is that vacuum drying requires more specialized equipment to achieve the necessary preservation of the material's architecture.
Balancing Time and Temperature
A vacuum oven allows for faster drying than air drying, but it must still be controlled.
Setting the vacuum too high or the temperature above the recommended 60 degrees Celsius can still damage the delicate MOF structure.
Operators must balance the vacuum strength with temperature to ensure solvent removal does not compromise the precursor's integrity.
Making the Right Choice for Your Goal
The use of a vacuum drying oven is a non-negotiable step for high-quality ZIF-8 synthesis.
- If your primary focus is Structural Integrity: Adhere strictly to the 60°C limit under vacuum to prevent pore collapse and ensure the MOF framework remains robust.
- If your primary focus is Material Purity: Utilize the vacuum to ensure deep extraction of ethanol residues, preventing solvent contamination in downstream applications.
By controlling the atmosphere and temperature simultaneously, you ensure the ZIF-8 precursor remains chemically stable and structurally sound.
Summary Table:
| Feature | Vacuum Drying (60°C) | Standard Drying | Benefit for ZIF-8 |
|---|---|---|---|
| Mechanism | Lowered boiling point via vacuum | Convective heat transfer | Prevents thermal degradation |
| Structural Impact | Preserves delicate MOF pores | Risk of framework collapse | Maintains high surface area |
| Solvent Removal | Deep extraction of ethanol/H2O | Surface-level evaporation | Ensures high material purity |
| Physical State | Loose powder (low agglomeration) | Potential clumping/agglomeration | Easier downstream processing |
Precision is critical when synthesizing sensitive materials like ZIF-8. Backed by expert R&D and manufacturing, KINTEK offers specialized vacuum, muffle, tube, and CVD systems designed to protect your material’s structural integrity. Whether you need standard lab high-temp furnaces or fully customizable solutions for unique research needs, our equipment ensures consistent, high-quality results for your laboratory. Contact KINTEK today to discover how our advanced vacuum drying solutions can optimize your MOF synthesis and prevent thermal collapse.
Visual Guide
References
- Jiuyu Chen, Zhiwen Liu. Cu0-Functionalized, ZIF-8-Derived, Nitrogen-Doped Carbon Composites for Efficient Iodine Elimination in Solution. DOI: 10.3390/nano15020105
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
People Also Ask
- What is the significance of vacuum furnaces in powder metallurgy? Achieve High-Purity, Dense Metal Parts
- What is the purpose of using a vacuum oven for Fe-N-C precursor synthesis? Optimize Catalyst Structural Integrity
- How do industrial-grade vacuum furnaces refine grain and relieve stress in Inconel 718? Achieve Peak Superalloy Strength
- What is a graphitization furnace? Unlocking Superior Graphite Properties for Your Industry
- What are the benefits of using graphite heating elements in vacuum furnaces? Achieve Extreme Heat and Durability
- What are some applications of graphite materials in vacuum furnace processing? Discover Key Uses and Benefits
- What is a vacuum furnace and how does it differ from an atmosphere furnace? Choose the Right Heat Treatment for Your Lab
- What is the significance of vacuum brazing in modern manufacturing? Achieve Strong, Pure Joints for Critical Applications



















