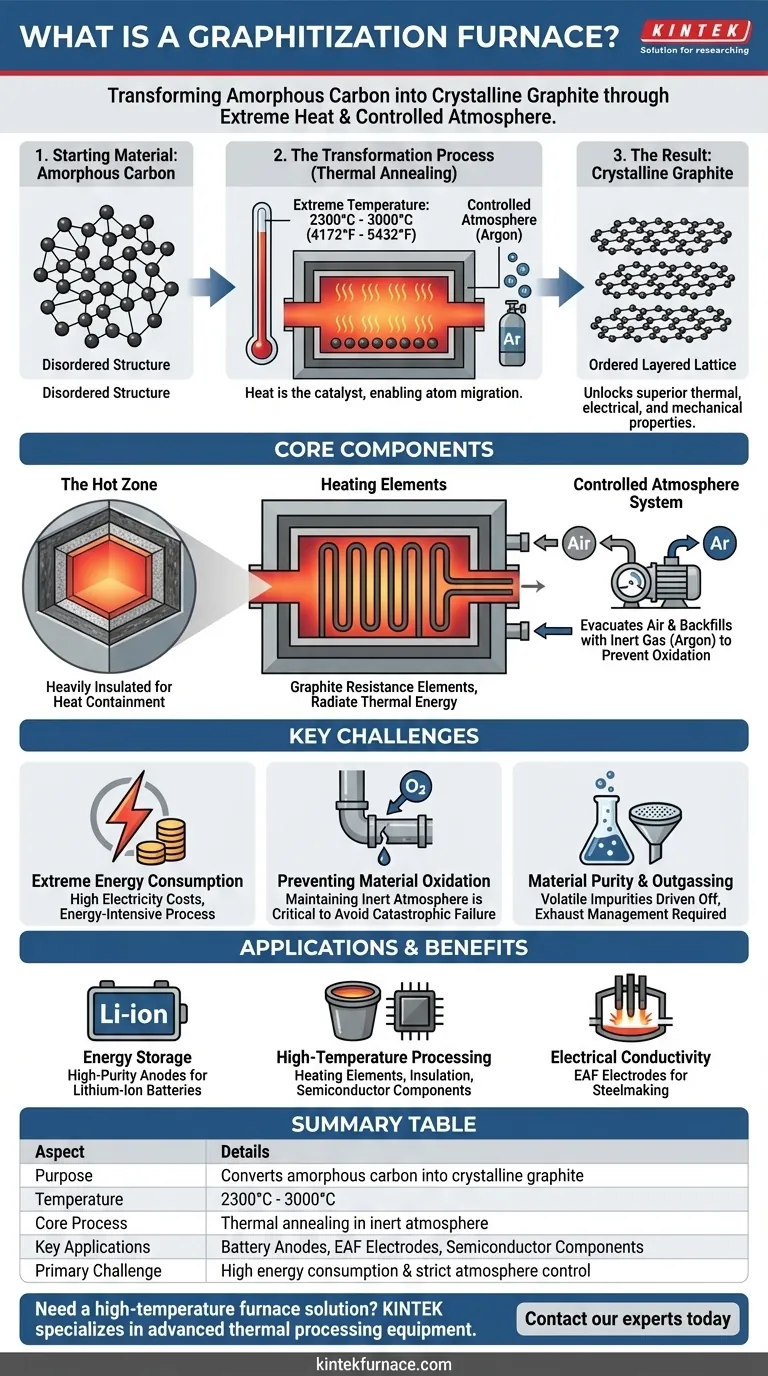
At its core, a graphitization furnace is a specialized piece of industrial equipment designed for a profound material transformation. It converts amorphous, or structurally disordered, carbon-based materials into highly ordered, crystalline graphite. This is achieved by subjecting the material to extremely high temperatures, typically ranging from 2300°C (4172°F) to 3000°C (5432°F) in a controlled atmosphere.
The purpose of a graphitization furnace is not merely to heat a material, but to fundamentally re-engineer its atomic structure. By forcing carbon atoms into an ordered, layered lattice, the furnace unlocks the superior thermal, electrical, and mechanical properties of synthetic graphite that are critical for modern industry.

How the Transformation Occurs
Graphitization is a process of thermal annealing taken to an extreme. The intense heat provides the energy needed for carbon atoms to break their disordered bonds and rearrange into a more stable, crystalline state.
The Starting Material: Amorphous Carbon
The input is typically a pre-formed carbon material, often derived from petroleum coke or coal tar pitch. At a microscopic level, its carbon atoms are arranged randomly, lacking the long-range order that defines a crystal.
The Catalyst: Extreme Temperature
Heat is the sole catalyst. As the furnace temperature rises above 2200°C, the carbon atoms gain enough kinetic energy to overcome their structural inertia. They begin to migrate and re-orient themselves.
The Result: Crystalline Graphite
Upon reaching and holding temperatures up to 3000°C, the atoms settle into graphite’s signature hexagonal lattice. This structure consists of strongly bonded layers (graphene sheets) that are weakly bonded to each other, giving graphite its unique properties.
The Core Components of a Graphitization Furnace
While designs vary, these furnaces share several critical components necessary to achieve and withstand such extreme conditions.
The Hot Zone
This is the heart of the furnace, containing the material being processed. It is heavily insulated with materials like graphite felt or carbon fiber composites (CFC) to contain the immense heat and minimize energy loss.
The Heating Elements
The heating itself is typically accomplished using large graphite resistance elements. An enormous electrical current is passed through these elements, which glow white-hot and radiate thermal energy throughout the hot zone.
The Controlled Atmosphere System
Operating at these temperatures in the presence of oxygen would cause the carbon material to instantly oxidize (burn). To prevent this, the furnace chamber is first evacuated of air and then backfilled with an inert gas, almost always argon. This inert atmosphere is maintained throughout the heating cycle.
Understanding the Trade-offs and Challenges
The production of synthetic graphite is a powerful but demanding process involving significant operational complexities.
Extreme Energy Consumption
Heating a large furnace to 3000°C is incredibly energy-intensive. The cost of electricity is a major factor in the economic viability of graphitization, making it an expensive and resource-heavy process.
Preventing Material Oxidation
Maintaining the integrity of the inert atmosphere is the single most critical operational challenge. Any leak that allows air (oxygen) to enter the hot zone at temperature will result in catastrophic failure, destroying both the product and potentially the furnace internals.
Material Purity and Outgassing
The process itself drives off many volatile impurities from the initial carbon material, which is a key benefit for creating high-purity graphite. However, these outgassed substances must be managed and scrubbed from the furnace exhaust.
How to Apply This to Your Project
The decision to use synthetic graphite is driven entirely by the need for properties that cannot be met by other materials. The graphitization process is what creates these properties.
- If your primary focus is energy storage: The high purity and controlled crystalline structure of synthetic graphite make it the dominant material for manufacturing high-performance anodes in lithium-ion batteries.
- If your primary focus is high-temperature processing: The resulting material is used to create heating elements, insulation, and crucibles for the semiconductor industry and other metallurgical applications.
- If your primary focus is electrical conductivity: Graphite electrodes for electric arc furnaces (EAF) in steelmaking are a major application, leveraging the material's unique ability to handle massive electrical currents without melting.
Ultimately, the graphitization furnace is a critical industrial tool that creates an advanced material foundational to modern energy, electronics, and manufacturing.
Summary Table:
| Key Aspect | Details |
|---|---|
| Purpose | Converts amorphous carbon into crystalline graphite |
| Temperature Range | 2300°C to 3000°C (4172°F to 5432°F) |
| Core Process | Thermal annealing in a controlled, inert atmosphere |
| Key Applications | Lithium-ion battery anodes, EAF electrodes, semiconductor components |
| Primary Challenge | High energy consumption and strict atmosphere control |
Need a high-temperature furnace solution tailored to your unique requirements?
KINTEK specializes in advanced thermal processing equipment. Leveraging our exceptional R&D and in-house manufacturing, we provide diverse laboratories and industrial facilities with robust high-temperature furnace solutions. Our product line, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, is complemented by strong deep customization capabilities to precisely meet your specific graphitization or other high-temperature experimental needs.
Contact our experts today to discuss how we can enhance your material processing capabilities.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What is the primary function of a muffle furnace for BaTiO3? Master High-Temp Calcination for Ceramic Synthesis
- What role does a muffle furnace play in the preparation of MgO support materials? Master Catalyst Activation
- What is the key role of a muffle furnace in the pretreatment of boron sludge and szaibelyite? Unlock Higher Process Efficiency
- What environmental conditions are critical for SiOC ceramicization? Master Precise Oxidation & Thermal Control
- What is the role of a muffle furnace in the study of biochar regeneration and reuse? Unlock Sustainable Water Treatment



















