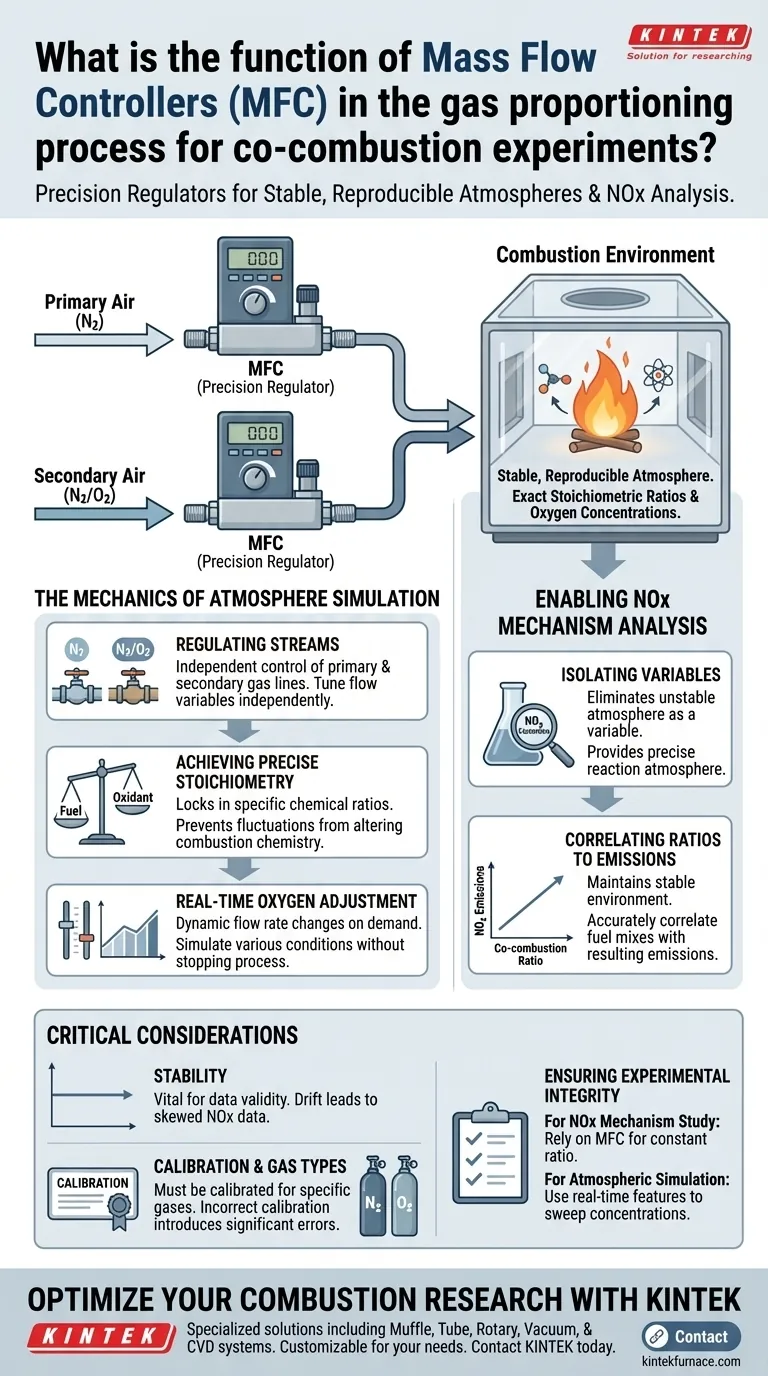
Mass Flow Controllers (MFCs) serve as the precision regulators within the gas proportioning system of co-combustion experiments. Their primary function is to manage the flow rates of primary air gases (typically nitrogen) and secondary air mixtures (nitrogen and oxygen) in real-time, ensuring the combustion environment matches exact experimental specifications.
By enabling the rigorous control of oxygen concentrations and chemical stoichiometric ratios, MFCs provide the stable, reproducible atmospheres required to isolate and study complex NOx formation mechanisms.

The Mechanics of Atmosphere Simulation
Regulating Primary and Secondary Streams
In co-combustion setups, gas delivery cannot be static. MFCs are tasked with the dynamic management of distinct gas lines.
They independently control the primary air, which is often an inert gas like nitrogen, and the secondary air, which usually introduces the oxidant (oxygen) mixed with nitrogen. This separation allows for independent tuning of flow variables.
Achieving Precise Stoichiometry
The core value of an MFC is its ability to lock in a specific chemical stoichiometric ratio.
By strictly regulating the ratio of carrier gases to oxidants, the controller ensures that the fuel reacts under exact, calculated conditions. This prevents fluctuations in gas supply from altering the combustion chemistry during the experiment.
Real-Time Oxygen Adjustment
Experimental conditions often require shifting parameters. MFCs allow for real-time adjustment of flow rates.
This capability enables researchers to simulate specific oxygen concentrations on demand. It allows the experiment to mimic various industrial boiler conditions or theoretical scenarios without stopping the process to manually re-calibrate valves.
Enabling NOx Mechanism Analysis
Isolating Variables
To understand how Nitrogen Oxides (NOx) form, researchers must rule out environmental inconsistencies.
If the gas flow fluctuates, it becomes impossible to tell if changes in NOx emissions are due to the co-combustion fuel ratio or simply an unstable atmosphere. MFCs eliminate this variable by providing a precise reaction atmosphere.
Correlating Ratios to Emissions
The ultimate goal of using MFCs in this context is to study NOx formation mechanisms.
By maintaining a stable environment, researchers can accurately correlate different co-combustion ratios (the mix of fuels) with the resulting emissions. This data is essential for optimizing fuel blends to minimize pollution.
Critical Considerations for Accuracy
The Necessity of Stability
While the primary focus is on setting a rate, the implicit requirement is stability.
Just as in material synthesis where gas stability influences crystal growth, in combustion, a stable gas environment is vital for data validity. Any drift in the MFC's calibration can lead to unintended "supersaturation" of oxygen or fuel-rich zones, skewing the NOx data.
Calibration and Gas Types
It is critical to note that MFCs must be calibrated for the specific gases being used (Nitrogen vs. Oxygen).
Using a controller calibrated for nitrogen to measure oxygen can introduce significant errors in the flow rate reading. This discrepancy would directly impact the calculated stoichiometric ratio and invalidates the simulation of specific oxygen concentrations.
Ensuring Experimental Integrity
To derive meaningful data from your co-combustion experiments, apply the following principles:
- If your primary focus is NOx Mechanism Study: Rely on the MFC to maintain a constant, unwavering stoichiometric ratio to isolate the chemical impact of your fuel blend.
- If your primary focus is Atmospheric Simulation: Utilize the real-time adjustment features to sweep through specific oxygen concentrations, mapping how the reaction changes across different air-to-fuel regimes.
Precision in gas delivery is not just a logistical detail; it is the foundation upon which accurate combustion chemistry analysis is built.
Summary Table:
| Feature | Function in Co-Combustion | Impact on Research |
|---|---|---|
| Stream Regulation | Independent control of primary (N2) and secondary (N2/O2) air. | Allows for isolated tuning of flow variables. |
| Stoichiometry | Locks in specific chemical stoichiometric ratios. | Prevents fluctuations from altering combustion chemistry. |
| Real-Time Tuning | Dynamic adjustment of oxygen concentrations. | Simulates various industrial boiler conditions on demand. |
| Flow Stability | Eliminates drift in gas supply during experiments. | Isolates fuel impact for accurate NOx mechanism analysis. |
Optimize Your Combustion Research with KINTEK
Precise atmospheric control is the backbone of valid NOx emission data. Backed by expert R&D and manufacturing, KINTEK offers specialized laboratory solutions including Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to integrate seamlessly with your gas proportioning needs.
Whether you are mapping stoichiometric ratios or simulating complex industrial atmospheres, our high-temperature furnaces provide the stability your research demands. Contact KINTEK today to discuss how our customizable systems can enhance the integrity of your combustion experiments.
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Cylindrical Resonator MPCVD Machine System for Lab Diamond Growth
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Kiln for Pyrolysis Plant Heating
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
People Also Ask
- Why must rare earth-based halide solid electrolytes be handled in a glove box? Protect Your Materials from Degradation
- What is the maximum vacuum capacity of the water circulating vacuum pump? Uncover Its Ideal Lab Applications
- What additional convenience feature is included with the water circulating vacuum pump? Discover Easy Mobility and More
- How does a precision programmed cooling system influence the structural integrity of Al2O3-TiC composite materials?
- Why is a copper getter chamber integrated into heating systems? Ensure Ultra-Pure Alloy Processing
- Why is a precise gas flow control and supply system necessary during the thermochemical conversion of rice husk biochar?
- What is the sucking rate for a single tap on the water circulating vacuum pump? Get Key Specs for Your Lab
- Why Is a High-Precision Heating and Stirring Platform Necessary for ZnO Sol-Gel Synthesis? Achieve Perfect Nanoparticles



















