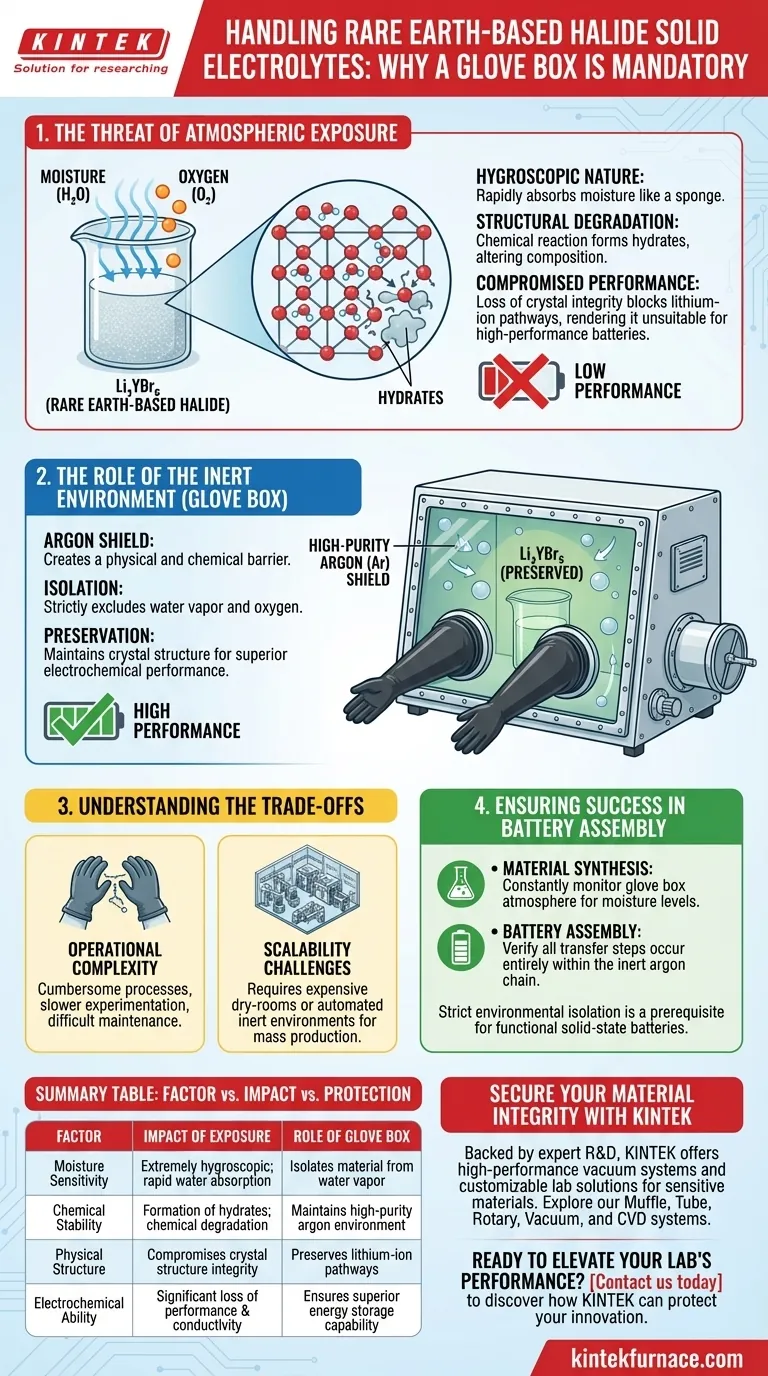
Rare earth-based halide solid electrolytes require a glove box because they are fundamentally unstable in ambient conditions. Materials such as Li3YBr6 are extremely hygroscopic, meaning they rapidly absorb moisture from the air, causing immediate chemical degradation and the formation of hydrates.
The glove box acts as a critical barrier, providing a high-purity argon environment that isolates the electrolyte from water and oxygen to preserve the material's crystal structure and electrochemical capability.

The Threat of Atmospheric Exposure
The Hygroscopic Nature of Halides
Rare earth-based halide electrolytes possess a high affinity for moisture.
When exposed to standard air, even for brief periods, these materials act like sponges. They attract and absorb water molecules from the environment, a property known as being extremely hygroscopic.
Structural Degradation and Hydrate Formation
The absorption of moisture is not a passive event; it triggers a chemical reaction.
This reaction leads to the formation of hydrates, effectively altering the chemical composition of the electrolyte. This transformation compromises the integrity of the crystal structure, which is the pathway through which lithium ions move.
Impact on Electrochemical Performance
The ultimate casualty of this degradation is the performance of the battery.
Once the crystal structure is altered by moisture or oxygen, the material cannot deliver the superior electrochemical performance required for effective energy storage. The material becomes unsuitable for use in all-solid-state lithium batteries.
The Role of the Inert Environment
Creating an Argon Shield
A glove box provides a controlled, hermetically sealed environment.
It is typically filled with high-purity argon, an inert gas that does not react with the halide electrolytes. This creates a physical and chemical "shield" around the material during handling and storage.
Isolating from Oxygen and Water
The primary function of this environment is total isolation.
By strictly excluding water vapor and oxygen, the glove box prevents the degradation mechanisms described above. This allows researchers to manipulate the material without fear of immediate hydration or oxidation.
Understanding the Trade-offs
Operational Complexity
While necessary, the requirement for a glove box introduces significant friction to the workflow.
Processes that would be simple on a benchtop become cumbersome when performed through thick rubber gloves. This limits the speed of experimentation and makes equipment maintenance more difficult.
Scalability Challenges
The sensitivity of these materials poses a challenge for mass manufacturing.
Scaling up production from a laboratory glove box to a factory floor requires sophisticated, expensive dry-room facilities or automated inert environments. This adds cost and engineering complexity to the commercialization of halide-based solid-state batteries.
Ensuring Success in Battery Assembly
To maximize the potential of rare earth-based halide electrolytes, you must adhere to strict environmental controls.
- If your primary focus is material synthesis: Ensure your glove box atmosphere is constantly monitored for moisture levels to prevent invisible degradation during reaction phases.
- If your primary focus is battery assembly: Verify that all transfer steps between synthesis and cell fabrication occur entirely within the inert argon chain to maintain ionic conductivity.
Strict environmental isolation is not merely a precaution; it is the prerequisite for functional high-performance solid-state batteries.
Summary Table:
| Factor | Impact of Atmospheric Exposure | Role of Glove Box Protection |
|---|---|---|
| Moisture Sensitivity | Extremely hygroscopic; rapid water absorption | Isolates material from water vapor |
| Chemical Stability | Formation of hydrates; chemical degradation | Maintains high-purity argon environment |
| Physical Structure | Compromises crystal structure integrity | Preserves lithium-ion pathways |
| Electrochemical Ability | Significant loss of performance & conductivity | Ensures superior energy storage capability |
Secure Your Material Integrity with KINTEK
Maintaining the delicate stability of rare earth-based halide electrolytes requires more than just standard tools—it demands precision-engineered environments. Backed by expert R&D and manufacturing, KINTEK offers high-performance vacuum systems and customizable lab solutions designed to protect your most sensitive materials from oxygen and moisture.
Whether you are scaling up synthesis or refining battery assembly, our range of Muffle, Tube, Rotary, Vacuum, and CVD systems are tailored to meet the unique needs of solid-state battery researchers and manufacturers.
Ready to elevate your lab's performance? Contact us today to discover how KINTEK can protect your innovation.
Visual Guide
References
- Zhichao Zeng, Yaping Du. Vacuum evaporation-assisted reaction: sustainable solution for application of rare earth-based halide solid-state electrolytes. DOI: 10.1039/d5sc00003c
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the primary function of a high-alumina powder crucible? Ensure Purity in Maraging Steel Pre-treatment
- What is the function of a laboratory pellet press in PCM preparation? Optimize Building Energy Storage Materials
- Why is a high-purity graphite crucible essential for magnesium vacuum distillation? Achieve Maximum Purity & Efficiency
- What are the functions of a boron nitride (BN) crucible and internal packing powder? Optimize Si3N4 Sintering Now
- Why is a graphite crucible used for melting Al-Mg-Si alloys? Superior Purity & Thermal Efficiency
- Why are high-purity alumina crucibles utilized for CsV3Sb5 crystal growth? Ensure Purity in Self-Flux Synthesis
- How do cooling modules in high-temperature laboratory furnaces manage thermal energy? Protect Your System Components
- What are the alternative names for a Laboratory Furnace? Find the Right High-Temperature Tool for Your Lab




