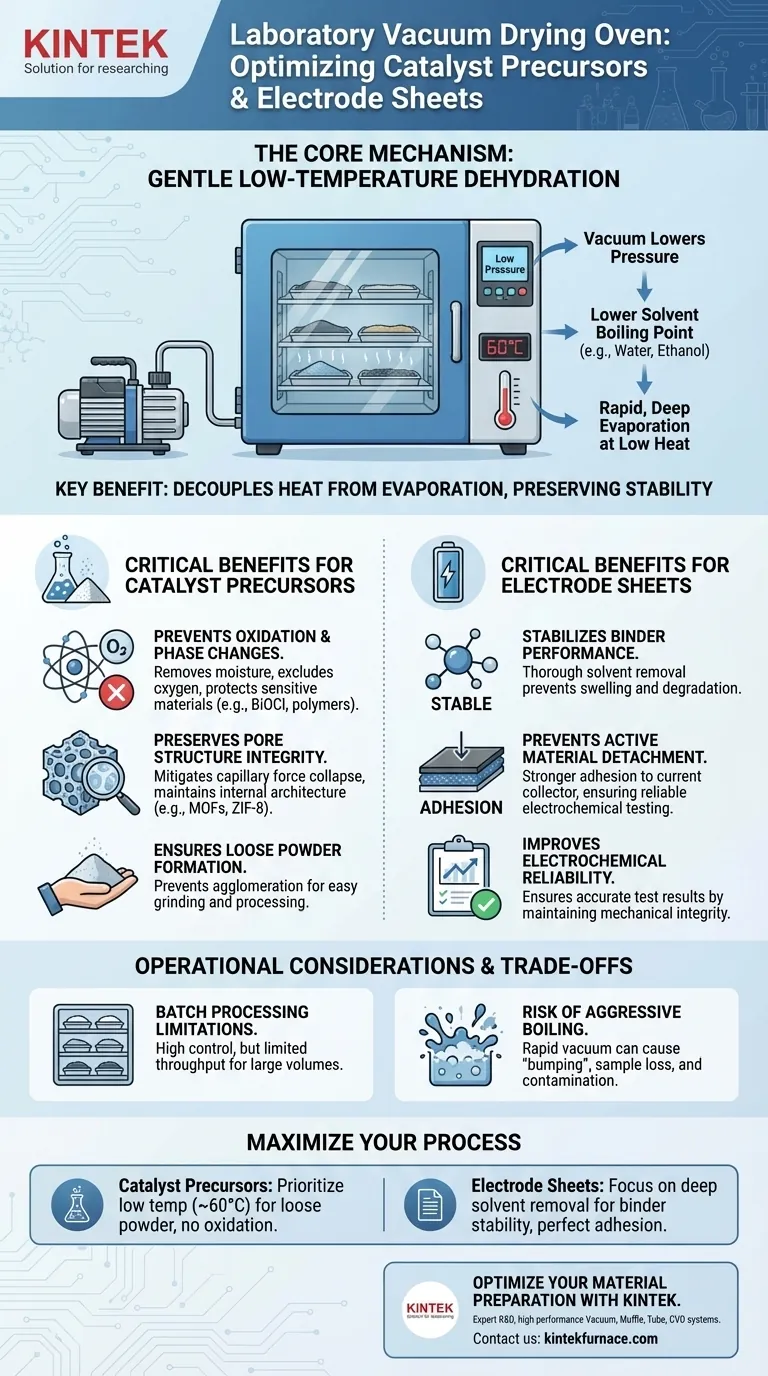
A laboratory vacuum drying oven functions by creating a low-pressure environment that significantly lowers the boiling point of solvents. This allows for the rapid and deep removal of residual solvents—such as methanol, water, or mixed alcohols—from the pores of materials without subjecting them to damagingly high temperatures.
The core value of vacuum drying lies in its ability to decouple heat from evaporation. By drying materials at lower temperatures under vacuum, you preserve the chemical stability of binders and the structural integrity of delicate porous precursors, ensuring accurate performance in downstream electrochemical applications.

The Mechanism: Low-Temperature Dehydration
Lowering the Boiling Point
The fundamental advantage of this equipment is the manipulation of atmospheric pressure. By reducing the pressure inside the chamber, the boiling point of solvents is lowered.
Gentle Thermal Treatment
This allows stubborn solvents like water and ethanol to evaporate efficiently at temperatures as low as 60°C or 70°C. This is critical for materials that might degrade, oxidize, or undergo unwanted phase changes at standard boiling temperatures (100°C+).
Critical Benefits for Catalyst Precursors
Preventing Oxidation and Phase Changes
Many catalyst precursors, such as Bi2SiO5, BiOCl, and various polymers, are sensitive to heat and oxygen. Vacuum drying removes moisture while simultaneously excluding oxygen.
This prevents the oxidation of the material and avoids thermally induced phase transitions that could alter the catalyst's intended properties before calcination or sintering.
Preserving Pore Structure Integrity
For porous materials like Metal-Organic Frameworks (e.g., ZIF-8) or Carbon Molecular Sieve precursors, structural collapse is a major risk during drying.
Rapid high-temperature drying can cause capillary forces to collapse delicate pores. Vacuum drying mitigates this, ensuring the internal pore architecture remains intact for subsequent surface area utilization.
Ensuring Loose Powder Formation
Drying wet precipitates often leads to hard agglomerates that are difficult to process. Vacuum drying helps maintain precursors in a loose, powdery state.
By preventing severe agglomeration during the solvent removal phase, the material remains easy to grind and process, ensuring better fluidity for subsequent steps like calcination.
Critical Benefits for Electrode Sheets
Stabilizing Binder Performance
In electrode fabrication, the interaction between the active material and the binder is crucial. Vacuum drying thoroughly removes solvents from the coating.
This ensures the long-term stability of the binder, preventing it from swelling or degrading due to trapped solvent residues.
Preventing Active Material Detachment
Residual solvents within the electrode pores can weaken the adhesion of the active material to the current collector.
By eliminating these residues, the vacuum oven prevents the detachment of active materials. This directly correlates to the reliability and accuracy of electrochemical testing results, as the electrode structure remains mechanically sound.
Operational Considerations and Trade-offs
Batch Processing Limitations
Unlike conveyor-belt drying systems, laboratory vacuum ovens are typically batch-process units. This provides high control over specific variables but may limit throughput for larger sample volumes compared to continuous industrial methods.
The Risk of aggressive Boiling
While low pressure aids drying, applying a vacuum too quickly to a very wet sample can cause "bumping" or violent boiling. This can splatter the material inside the chamber, leading to sample loss or cross-contamination if not managed by gradually lowering the pressure.
Making the Right Choice for Your Goal
To maximize the utility of your vacuum drying process, align your parameters with your specific material needs:
- If your primary focus is Catalyst Precursors: Prioritize low temperatures (e.g., 60°C) to prevent oxidation and ensure the powder remains loose for easy grinding.
- If your primary focus is Electrode Sheets: Focus on the thoroughness of solvent removal to guarantee binder stability and prevent active material detachment during testing.
Success in material preparation relies not just on removing moisture, but on removing it without altering the fundamental architecture of your sample.
Summary Table:
| Feature | Benefit for Catalyst Precursors | Benefit for Electrode Sheets |
|---|---|---|
| Low-Pressure Environment | Lowers solvent boiling point to prevent thermal degradation. | Ensures deep removal of residual solvents from thick coatings. |
| Oxygen Exclusion | Prevents oxidation of sensitive materials (e.g., BiOCl). | Protects current collectors and active materials from corrosion. |
| Gentle Dehydration | Preserves delicate pore structures (MOFs/ZIFs) and prevents clumping. | Maintains binder stability and prevents active material detachment. |
| Temperature Control | Facilitates loose powder formation for easier post-processing. | Improves adhesion and reliability of electrochemical testing results. |
Optimize Your Material Preparation with KINTEK
Don't compromise the structural integrity of your catalysts or the performance of your electrode sheets. At KINTEK, we understand that precision starts with the right thermal environment.
Backed by expert R&D and manufacturing, we offer high-performance Vacuum, Muffle, Tube, and CVD systems—all fully customizable to meet the unique demands of your laboratory. Whether you are preserving delicate MOF pores or ensuring perfect electrode adhesion, our equipment provides the stability and control your research requires.
Ready to elevate your lab's efficiency? Contact us today to find your custom heating solution!
Visual Guide
References
- Yulin Luo, Qi-Hui Wu. Carbon Nanotubes-Doped Metal Oxides and Metal Sulfides Heterostructure Achieves 3D Morphology Deposition of Li2S and Stable Long-Cycle Lithium–Sulfur Batteries. DOI: 10.3390/inorganics13060181
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What properties of a metal can be altered through vacuum heat treating? Enhance Strength, Ductility, and Corrosion Resistance
- Why is vacuum carburizing suitable for high-performance carburizing steels? Achieve Superior Hardening with Precision Control
- How are vacuum furnaces constructed and operated? Unlock Precision and Purity in Material Processing
- What is the basic working principle of a vacuum furnace? Unlock Pure, Controlled Heat Treatment
- What are the disadvantages of using a vacuum furnace? High Costs, Material Limits, and More
- What is Age Hardening in vacuum heat treating? Unlock Peak Metal Performance with Precision
- What conditions does a vacuum furnace provide for Nb3Sn repair? Precision Heat and High Vacuum for Superconductors
- How do computer-controlled systems enhance vacuum furnace operations? Achieve Precision and Repeatability in Heat Treatment



















