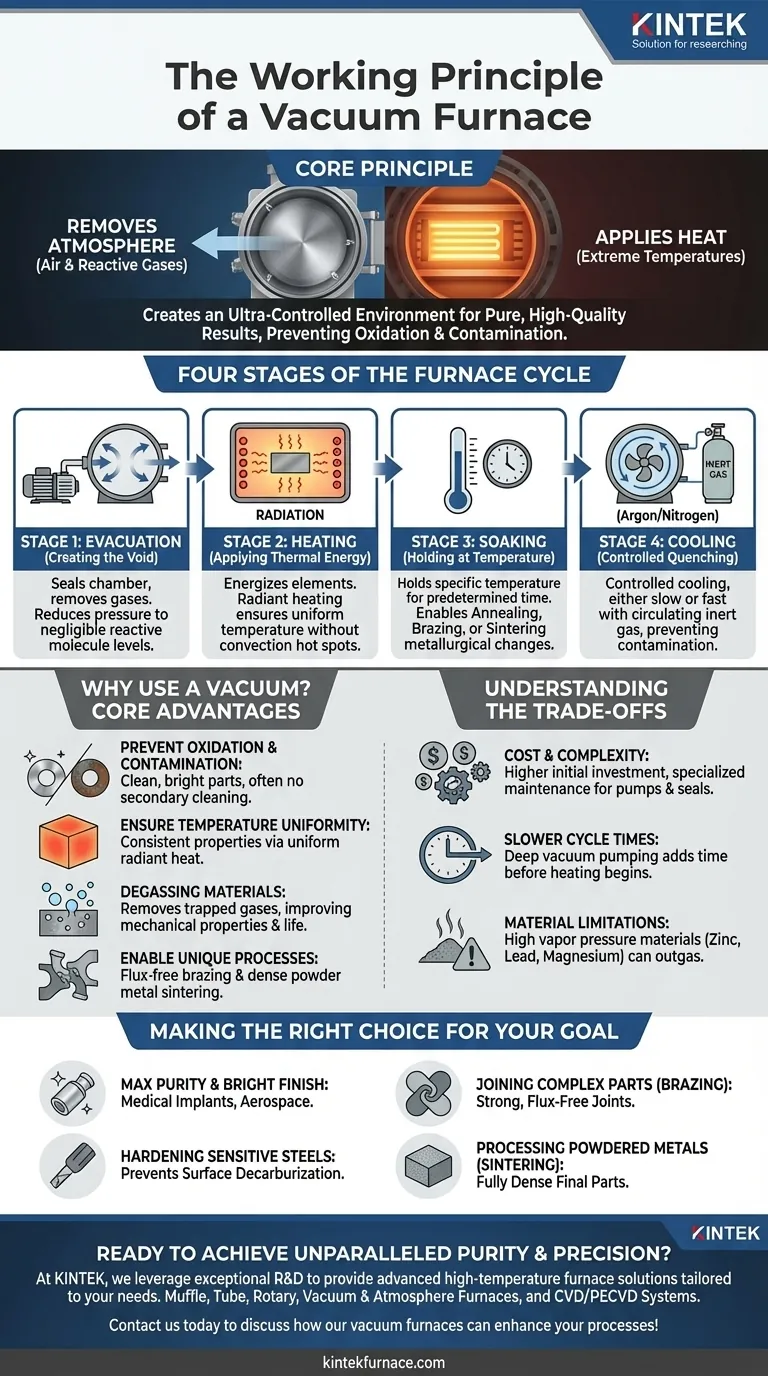
At its core, a vacuum furnace operates on a simple, powerful principle: it first removes the atmosphere from a sealed chamber and then applies heat. By creating a high-quality vacuum, the furnace eliminates air and other reactive gases. This allows materials like metals and ceramics to be heated to extreme temperatures without the risk of unwanted chemical reactions, such as oxidation or contamination, ensuring a pure and high-quality result.
The fundamental purpose of a vacuum furnace is not just to heat materials, but to create an ultra-controlled environment. By removing the reactive gases present in air, it enables heat treatment processes that are impossible to achieve otherwise, ensuring final material purity and structural integrity.

The Four Stages of a Vacuum Furnace Cycle
The operation of a vacuum furnace is not a single action but a precise, four-stage process. Understanding this cycle is key to understanding its capabilities.
Stage 1: Evacuation (Creating the Void)
The process begins by sealing the furnace chamber and activating a vacuum system. This system, typically involving one or more pumps, removes air and any other gases from the chamber.
The goal is to reduce the internal pressure to a level where the concentration of reactive molecules (like oxygen and water vapor) is negligible. This step is what prevents surface reactions on the workpiece.
Stage 2: Heating (Applying Thermal Energy)
Once the target vacuum level is reached, the heating elements are energized. These elements, often made of graphite or a refractory metal, transfer heat to the material primarily through radiation.
Because there is no air to transfer heat via convection, radiant heating in a vacuum can be exceptionally uniform. This prevents hot spots and ensures the entire workpiece reaches the target temperature consistently.
Stage 3: Soaking (Holding at Temperature)
The material is held at the specific target temperature for a predetermined amount of time. This "soaking" period is where the actual metallurgical process—such as annealing, brazing, or sintering—takes place.
The temperature control system is critical during this stage, maintaining the heat with extreme precision to ensure the desired changes in the material's microstructure are fully achieved.
Stage 4: Cooling (Controlled Quenching)
After the soaking stage is complete, the material must be cooled in a controlled manner. This can be done slowly by simply shutting off the heating elements and letting the furnace cool naturally under vacuum.
For faster cooling (quenching), an inert gas like argon or nitrogen can be backfilled into the chamber and circulated with a fan, rapidly removing heat without causing contamination.
Why Use a Vacuum? The Core Advantages
The complexity of a vacuum furnace is justified by the unique benefits it provides over traditional atmosphere furnaces.
Preventing Oxidation and Contamination
This is the primary reason to use a vacuum furnace. At high temperatures, most metals will readily react with oxygen, leading to scaling and a ruined surface finish. A vacuum eliminates this threat, resulting in clean, bright parts that often require no secondary cleaning.
Ensuring Temperature Uniformity
In a vacuum, the dominant mode of heat transfer is radiation. A well-designed furnace provides uniform radiant heat to all surfaces of the part, ensuring consistent properties throughout the material. This is difficult to achieve in atmosphere furnaces where convection currents can cause uneven heating.
Degassing Materials
Heating a material in a vacuum has the added benefit of pulling trapped gases (like hydrogen and oxygen) out from within the material itself. This degassing process can significantly improve the mechanical properties, density, and fatigue life of the final product.
Enabling Unique Processes
Certain advanced processes are only possible in a vacuum. For example, vacuum brazing allows for the joining of complex assemblies with exceptionally strong and clean joints without the need for corrosive fluxes. Likewise, sintering powdered metals in a vacuum is essential for creating dense, high-purity parts.
Understanding the Trade-offs
While powerful, vacuum furnaces are not the solution for every heating application. Objectivity requires acknowledging their limitations.
Cost and Complexity
Vacuum furnaces are significantly more expensive to purchase and operate than their atmospheric counterparts. The vacuum pumps, chamber seals, and sophisticated control systems require specialized maintenance and a higher initial investment.
Slower Cycle Times
The need to pump the chamber down to a deep vacuum level before heating can add considerable time to the overall process cycle. While modern pumps are fast, this evacuation step is an inherent part of the workflow.
Material Limitations
Some materials are not suitable for vacuum processing. Materials with high vapor pressures, such as zinc, lead, or magnesium, can "outgas" or evaporate at high temperatures under vacuum. This not only destroys the part but also contaminates the inside of the furnace.
Making the Right Choice for Your Goal
Selecting a vacuum furnace depends entirely on the required outcome for your material.
- If your primary focus is maximum purity and a bright finish (e.g., medical implants, aerospace components): The vacuum's ability to prevent any surface oxidation is non-negotiable.
- If your primary focus is joining complex parts (brazing): A vacuum furnace provides the ideal clean environment for brazing alloys to flow freely and create strong, flux-free joints.
- If your primary focus is hardening or treating sensitive tool steels: A vacuum prevents surface decarburization, a common defect in atmosphere furnaces that softens the steel's surface.
- If your primary focus is processing powdered metals (sintering): The vacuum is essential for removing binders and preventing oxidation, creating a strong, fully dense final part.
Ultimately, a vacuum furnace is a precision tool that provides you with ultimate control over the thermal processing environment.
Summary Table:
| Stage | Description | Key Process |
|---|---|---|
| Evacuation | Removes air and gases from the sealed chamber to create a vacuum. | Prevents surface reactions like oxidation. |
| Heating | Applies thermal energy via radiation for uniform temperature distribution. | Ensures consistent heating without hot spots. |
| Soaking | Holds material at target temperature for metallurgical changes. | Enables annealing, brazing, or sintering. |
| Cooling | Cools material slowly or rapidly with inert gas under controlled conditions. | Maintains purity and prevents contamination. |
Ready to achieve unparalleled purity and precision in your lab? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions tailored to your needs. Our product line includes Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all backed by strong deep customization capabilities to meet your unique experimental requirements. Contact us today to discuss how our vacuum furnaces can enhance your processes and deliver superior results for your materials!
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- What additional processes can a vacuum heat treatment furnace carry out? Unlock Advanced Material Processing
- What is the function of a vacuum sintering furnace in CoNiCrAlY coatings? Repairing Cold-Sprayed Microstructures
- What is the mechanism of a vacuum sintering furnace for AlCoCrFeNi2.1 + Y2O3? Optimize Your High-Entropy Alloy Processing
- How does vacuum heat treatment reduce workpiece deformation? Achieve Superior Dimensional Stability
- What is the role of the temperature control system in a vacuum furnace? Achieve Precise Material Transformations



















