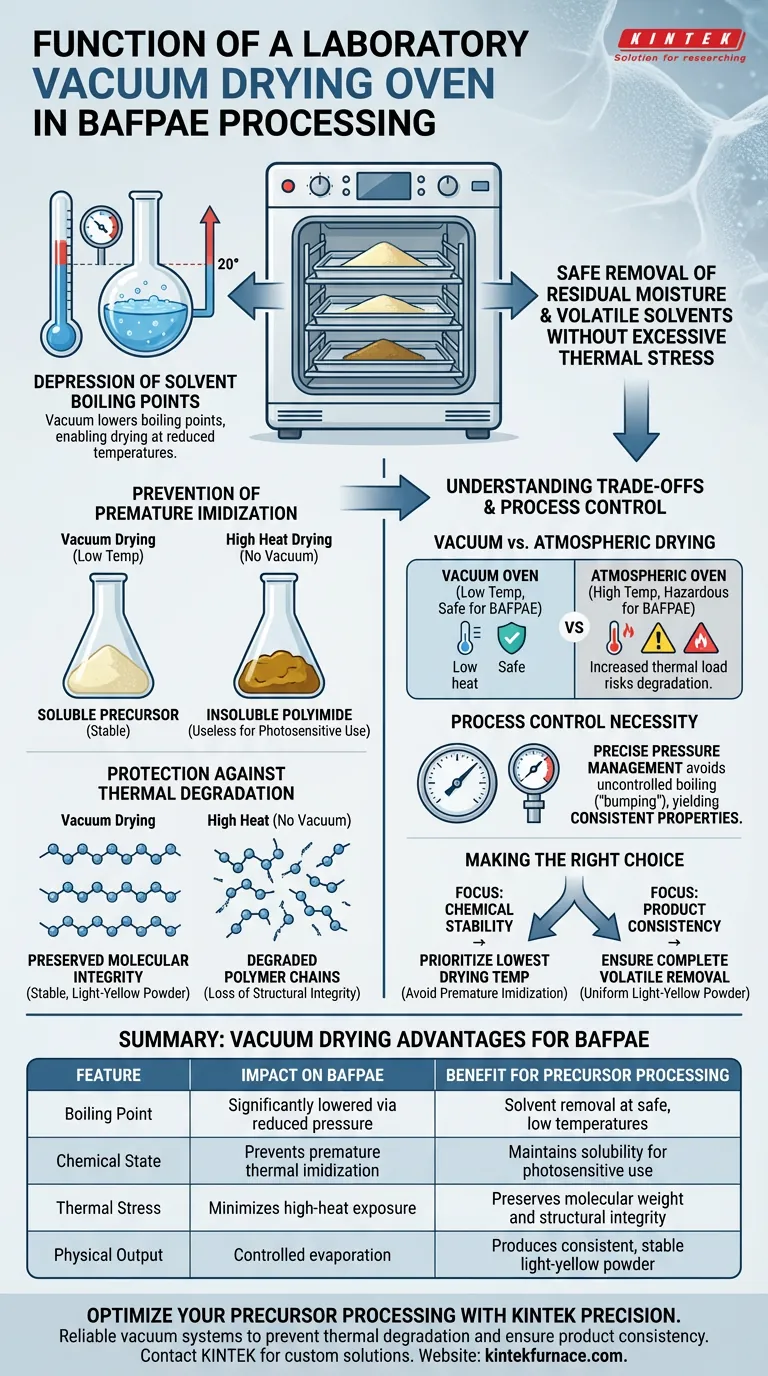
In the processing of the soluble photosensitive polyamide ester precursor (BAFPAE), the laboratory vacuum drying oven serves as a critical instrument for the safe removal of residual moisture and volatile solvents. By operating under reduced pressure, the oven facilitates the evaporation of these contaminants without subjecting the delicate precursor to excessive thermal stress.
The vacuum environment effectively lowers the boiling point of solvents, allowing for complete drying at reduced temperatures. This prevents premature thermal imidization or degradation, ensuring the precursor remains chemically stable.

The Critical Role of Vacuum Drying
Depression of Solvent Boiling Points
The fundamental advantage of this equipment is its ability to manipulate thermodynamics.
By creating a vacuum environment, the oven significantly reduces the boiling point of volatile solvents trapped within the BAFPAE matrix.
This allows the material to be dried thoroughly at temperatures far below what would be required at standard atmospheric pressure.
Prevention of Premature Imidization
BAFPAE is a precursor, meaning it is an intermediate material intended to react later in the process.
If exposed to high heat during the drying phase, the material may undergo premature thermal imidization.
This chemical change would convert the soluble precursor into an insoluble polyimide too early, rendering the material useless for its intended photosensitive applications.
Protection Against Thermal Degradation
Beyond imidization, high temperatures can cause general thermal degradation of the polymer chains.
Vacuum drying mitigates this risk entirely.
It preserves the molecular weight and structural integrity of the BAFPAE, resulting in a stable, light-yellow powder.
Understanding the Trade-offs
Vacuum vs. Atmospheric Drying
While standard drying ovens are effective for robust materials (such as catalyst supports where heat stabilizes spatial distribution), they are hazardous for BAFPAE.
Using a standard oven would require higher temperatures to remove the same amount of solvent.
This increased thermal load drastically increases the probability of degrading the precursor or triggering the curing reaction uncontrollably.
The Necessity of Process Control
The use of a vacuum oven introduces a requirement for precise pressure management.
If the vacuum is too strong, it could cause rapid boiling or "bumping" of the solvents, potentially altering the physical morphology of the powder.
However, when controlled correctly, it yields the most consistent physical and chemical properties achievable.
Making the Right Choice for Your Goal
To ensure the successful processing of BAFPAE, apply the following principles:
- If your primary focus is Chemical Stability: Prioritize vacuum levels that allow for the lowest possible drying temperature to completely avoid premature imidization.
- If your primary focus is Product Consistency: Ensure the drying cycle is long enough to remove all volatiles, resulting in a uniform light-yellow powder with predictable solubility.
The laboratory vacuum drying oven is not merely a drying tool; it is a preservation system essential for maintaining the reactivity and quality of the BAFPAE precursor.
Summary Table:
| Feature | Vacuum Drying Impact on BAFPAE | Benefit for Precursor Processing |
|---|---|---|
| Boiling Point | Significantly lowered via reduced pressure | Solvent removal at safe, low temperatures |
| Chemical State | Prevents premature thermal imidization | Maintains solubility for photosensitive use |
| Thermal Stress | Minimizes high-heat exposure | Preserves molecular weight and structural integrity |
| Physical Output | Controlled evaporation | Produces consistent, stable light-yellow powder |
Optimize Your Precursor Processing with KINTEK Precision
Maintaining the delicate chemical balance of BAFPAE requires rigorous thermal control and reliable vacuum performance. KINTEK provides industry-leading laboratory vacuum systems specifically engineered to prevent thermal degradation and ensure product consistency.
Backed by expert R&D and manufacturing, KINTEK offers Vacuum, Muffle, Tube, Rotary, and CVD systems, all fully customizable to meet your unique laboratory requirements. Whether you are scaling up polymer synthesis or refining sensitive precursors, our high-temp furnaces deliver the precision you need.
Ready to elevate your lab's efficiency and material quality? Contact KINTEK today to discuss your custom solution.
Visual Guide
References
- Soluble Photosensitive Polyimide Precursor with Bisphenol A Framework: Synthesis and Characterization. DOI: 10.3390/polym17111428
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- 1200℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What are the benefits of using electric actuators in this solution? Achieve Precision, Safety, and Efficiency in Automation
- How does a forced-air drying oven contribute to asphalt degradation? Accelerate Material Salt Erosion Simulation
- Why is a mixture of Argon (Ar) and Hydrogen (H2) required during beryl heat treatment? Master Color Transformation
- What is the purpose of treating EAF dust in a dryer? Ensure Precise Material Characterization & Data Integrity
- What is the technical necessity of heating and stirring for K-Na alloy anodes? Ensure Peak Battery Performance
- What role does graphite paper play in magnesium vapor condensation experiments? A Key to High-Purity Collection & Analysis
- How is induced heat generated in a conductive material exposed to a magnetic field? Master Rapid, Contactless Heating
- What is the role of an industrial oven in the drying stage of Rosa roxburghii biochar? Unlock Structural Integrity



















