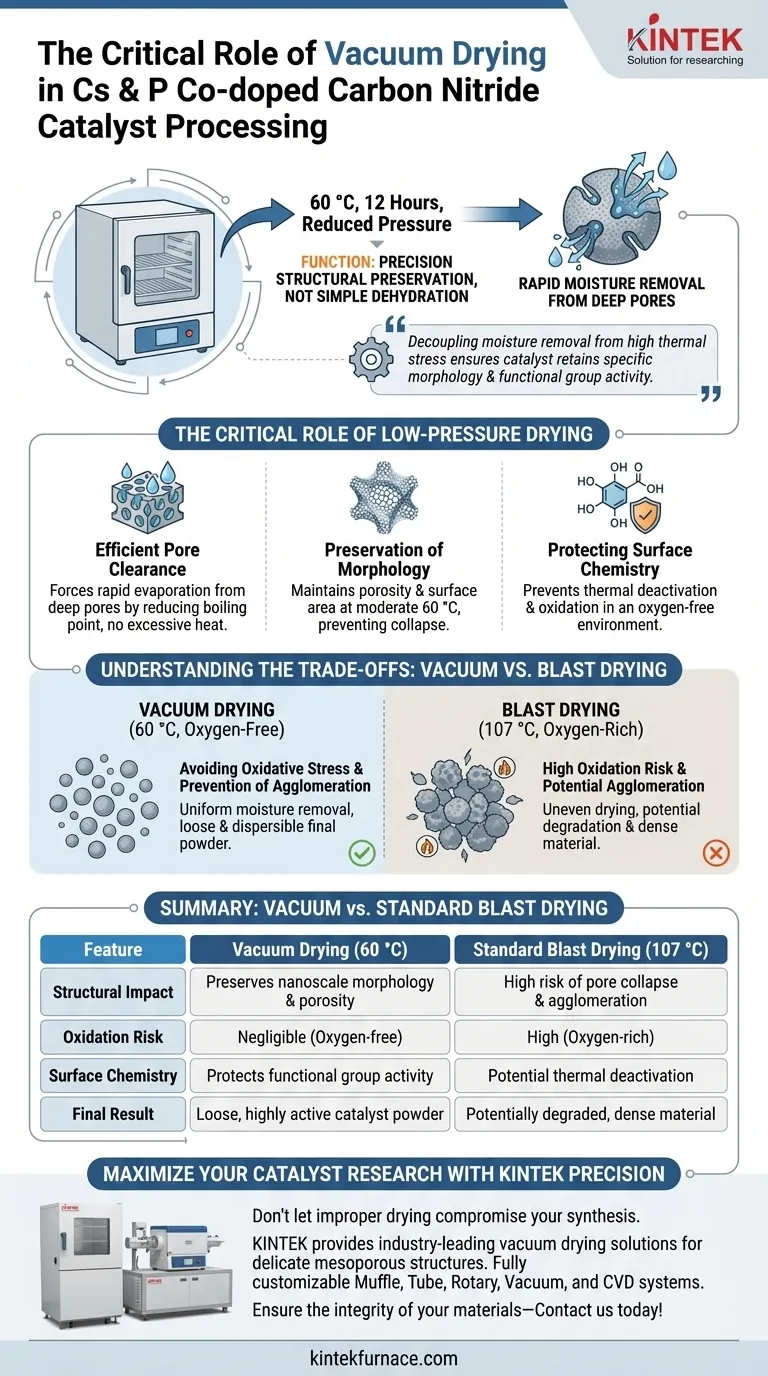
In the final processing stages of cesium and phosphorus co-doped carbon nitride catalysts, the laboratory vacuum drying oven functions as a precision tool for structural preservation rather than simple dehydration. Its primary role is to rapidly remove residual moisture from deep within the catalyst powder pores under a reduced-pressure environment—typically set at 60 °C for 12 hours—following ultrasonic dispersion and washing.
The vacuum drying process is essential for decoupling moisture removal from high thermal stress, ensuring the catalyst retains its specific surface morphology and functional group activity for accurate photocatalytic evaluation.

The Critical Role of Low-Pressure Drying
Efficient Pore Clearance
After washing and dispersion, moisture is often trapped deep within the mesoporous structure of the carbon nitride material.
Standard evaporation is inefficient here due to capillary forces. The vacuum oven reduces the boiling point of water, forcing rapid evaporation from these pores without requiring excessive heat.
Preservation of Morphology
Carbon nitride structures, particularly those co-doped with cesium and phosphorus, rely on specific nanoscale morphologies for their performance.
High-temperature drying can cause the collapse of these delicate structures. By operating at a moderate 60 °C, the vacuum oven maintains the material's porosity and surface area.
Protecting Surface Chemistry
The catalytic activity of co-doped carbon nitride is heavily dependent on specific surface functional groups.
Vacuum drying prevents the thermal deactivation of these groups. Furthermore, the absence of air prevents potential oxidation that might occur if the material were heated in an oxygen-rich environment.
Understanding the Trade-offs: Vacuum vs. Blast Drying
Avoiding Oxidative Stress
It is a common error to substitute a vacuum oven with a standard blast drying oven for this specific stage.
While a blast oven (often operating around 107 °C) allows for controlled solvent evaporation for precursors, exposing the final processed catalyst to high temperatures in air can degrade performance. The vacuum environment is strictly necessary to prevent oxidation during the final drying phase.
Prevention of Agglomeration
Drying at atmospheric pressure relies on hot air circulation, which can sometimes lead to uneven drying rates.
In contrast, vacuum drying ensures uniform moisture removal. This prevents the agglomeration of particles that can occur during uneven evaporation, ensuring the final powder remains loose and dispersible.
Making the Right Choice for Your Goal
To ensure your catalyst performs optimally during electrochemical or photocatalytic testing, consider the following processing parameters:
- If your primary focus is preserving active sites: Utilize the vacuum oven at 60 °C to prevent thermal deactivation of the doped functional groups.
- If your primary focus is structural integrity: Rely on the reduced-pressure environment to clear pores without risking the morphology collapse associated with high-heat air drying.
By prioritizing low-temperature vacuum extraction, you ensure the material you test represents the true potential of your synthesis strategy.
Summary Table:
| Feature | Vacuum Drying (60 °C) | Standard Blast Drying (107 °C) |
|---|---|---|
| Structural Impact | Preserves nanoscale morphology & porosity | High risk of pore collapse & agglomeration |
| Oxidation Risk | Negligible (Oxygen-free environment) | High (Oxygen-rich environment) |
| Surface Chemistry | Protects functional group activity | Potential thermal deactivation |
| Drying Mechanism | Low-pressure boiling point reduction | High-heat evaporation |
| Final Result | Loose, highly active catalyst powder | Potentially degraded, dense material |
Maximize Your Catalyst Research with KINTEK Precision
Don't let improper drying compromise your synthesis strategy. KINTEK provides industry-leading vacuum drying solutions designed to protect delicate mesoporous structures and sensitive functional groups.
Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you are working with co-doped carbon nitride or advanced nanomaterials, our lab high-temperature furnaces and ovens are fully customizable to meet your unique research requirements.
Ensure the integrity of your materials—Contact us today to find your perfect laboratory solution!
Visual Guide
References
- Juanfeng Gao, Youji Li. Synergistic Cs/P Co-Doping in Tubular g-C3N4 for Enhanced Photocatalytic Hydrogen Evolution. DOI: 10.3390/hydrogen6030045
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
People Also Ask
- How is furnace brazing utilized in the electronics and semiconductor industries? Master Precision Joining for High-Reliability Components
- What are the operational challenges associated with vacuum furnaces? Master Complexity for Superior Results
- Why is a vacuum oven necessary in the process flow for producing carbon nanospheres? Secure High Purity & Dispersion
- What are the benefits of low pressure carburizing in terms of metal quality? Boost Fatigue Strength and Reliability
- How does a high vacuum furnace ensure the purity of Ti-Nb alloys? Expert Guide to Sintering and Debinding
- What is the purpose of using an industrial vacuum resistance furnace? Enhancing Ti-33Mo-0.2C Alloy Performance
- What are the primary reasons for using movable material baskets to load scrap magnesium shavings into a vacuum sublimation furnace? Maximize Efficiency & Safety
- What advancements have been made in vacuum furnace energy efficiency and environmental impact? Discover Cleaner, More Efficient Heat Treatment



















