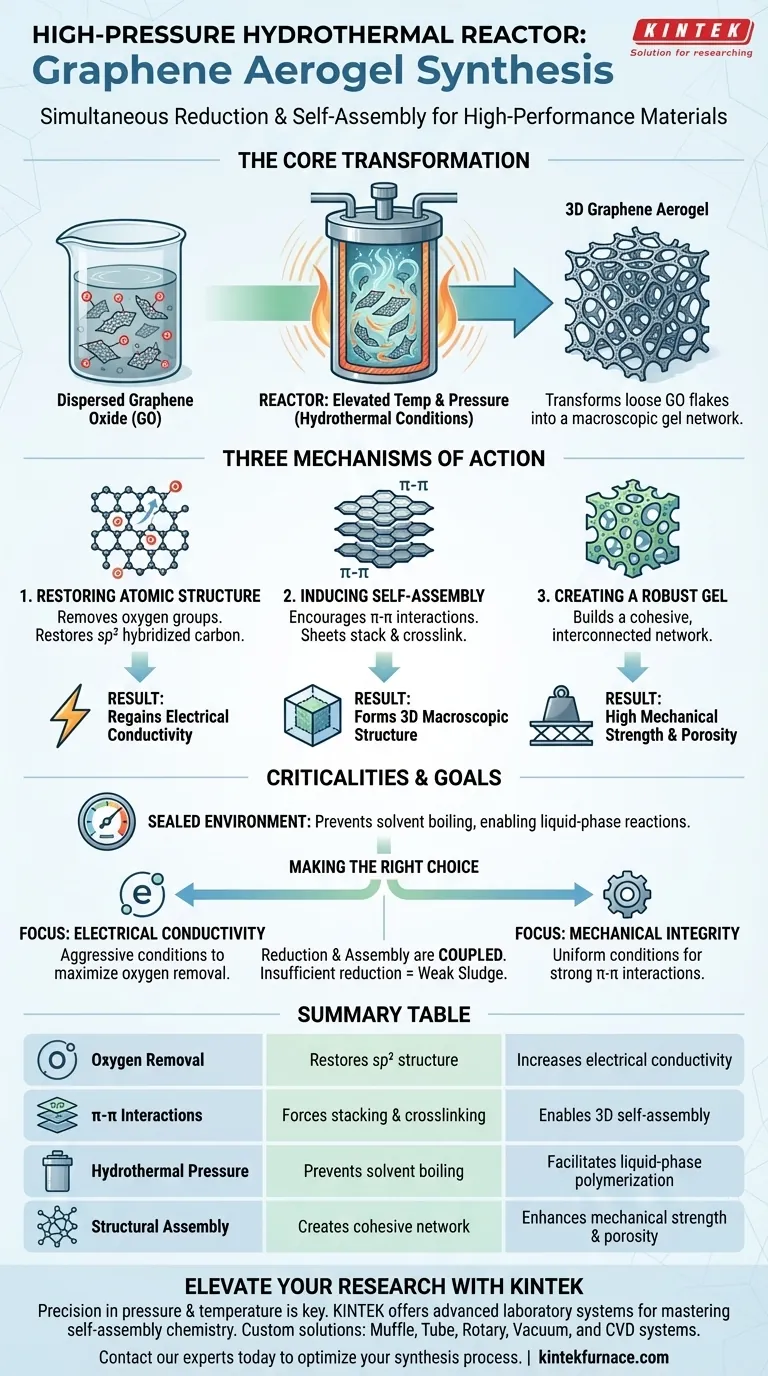
The high-pressure hydrothermal reactor serves as the critical vessel for simultaneously reducing graphene oxide and inducing structural self-assembly. By creating a sealed environment characterized by elevated temperature and pressure, the reactor forces dispersed graphene oxide (GO) sheets to shed oxygen atoms and physically interconnect into a unified three-dimensional framework.
The reactor's environment is the key driver that transforms loose, insulating GO flakes into a macroscopic gel network defined by high mechanical strength and restored electrical conductivity.

The Mechanism of Transformation
The synthesis of high-performance graphene carbon aerogels is not merely a drying process; it is a complex chemical and physical reconstruction. The reactor enables this through three specific mechanisms.
Restoring the Atomic Structure
Inside the reactor, the hydrothermal conditions facilitate the partial removal of oxygen-containing functional groups found on the surface of graphene oxide.
This removal is essential for restoring the $sp^2$ hybridized structure of the carbon atoms. This atomic restoration is the direct cause of the material regaining its conductive properties.
Inducing Physical Self-Assembly
As the oxygen groups are removed, the chemical nature of the graphene sheets changes.
The reactor's conditions encourage $\pi-\pi$ (pi-pi) interactions between the graphene sheets. This force causes the 2D sheets to spontaneously stack and crosslink, assembling themselves into a 3D macroscopic structure.
Creating a Robust Gel Network
The result of this self-assembly is a cohesive gel network rather than a powder or precipitate.
This network structure is responsible for the final material's high mechanical strength. It ensures the aerogel maintains its integrity and porosity, rather than collapsing back into a dense graphite-like solid.
Understanding the Criticalities
While the reactor enables synthesis, the process relies on maintaining a delicate balance of conditions within the sealed vessel.
The Role of the Sealed Environment
The reactor must remain perfectly sealed to maintain the necessary high pressure.
This pressure prevents the solvent (water) from boiling away at high temperatures, allowing "hydrothermal" reactions—such as dehydration and polymerization—to occur in a liquid-like high-density phase that would be impossible at atmospheric pressure.
The Link Between Reduction and Assembly
It is crucial to understand that reduction and assembly are coupled processes in this environment.
If the reactor conditions fail to sufficiently reduce the GO (remove oxygen), the $\pi-\pi$ interactions will remain too weak. This leads to a failure in self-assembly, resulting in a weak sludge rather than a strong, high-performance aerogel.
Making the Right Choice for Your Goal
When utilizing a high-pressure hydrothermal reactor for graphene aerogels, your specific performance metrics depend on how you manage the reduction process.
- If your primary focus is Electrical Conductivity: Ensure the reactor conditions (temperature and duration) are aggressive enough to maximize the removal of oxygen groups and fully restore the $sp^2$ structure.
- If your primary focus is Mechanical Integrity: Prioritize conditions that favor uniform self-assembly and strong $\pi-\pi$ interactions to build a robust 3D network.
The high-pressure reactor is not just a container; it is the active environment that dictates the final quality of your carbon aerogel.
Summary Table:
| Mechanism | Function in Synthesis | Impact on Final Aerogel |
|---|---|---|
| Oxygen Removal | Restores the $sp^2$ hybridized carbon structure | Increases electrical conductivity |
| $\pi-\pi$ Interactions | Forces 2D sheets to stack and crosslink | Enables 3D macroscopic self-assembly |
| Hydrothermal Pressure | Prevents solvent boiling at high temperatures | Facilitates liquid-phase polymerization |
| Structural Assembly | Creates a cohesive interconnected network | Enhances mechanical strength and porosity |
Elevate Your Nanomaterials Research with KINTEK
Precision in pressure and temperature is the difference between a weak sludge and a high-performance graphene aerogel. KINTEK provides the advanced high-pressure hydrothermal reactors and laboratory systems needed to master the complex chemistry of self-assembly.
Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to your specific research requirements. Whether you are aiming for maximum electrical conductivity or superior mechanical integrity, our equipment ensures the stable, sealed environment your materials demand.
Ready to optimize your synthesis process? Contact our laboratory experts today to find the perfect hydrothermal solution for your unique needs.
Visual Guide
References
- Yong Zhong, Xuguang Liu. Carbon Aerogel for Aqueous Phase Adsorption/Absorption: Application Performances, Intrinsic Characteristics, and Regulatory Constructions. DOI: 10.1002/sstr.202400650
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- Why use a precision oven for moxa floss samples? Ensure Accurate Air-Drying Basis for Combustion Research
- What is a batch furnace and how does it operate? Master Precision Heat Treatment for Diverse Applications
- How are high-temperature furnaces and precision balances used for alloy oxidation kinetics? Expert Analysis
- Why is the initial concentration of siloxane systems performed in a vacuum oven? Achieve Defect-Free Material Curing
- Why is the enhancement of coke strength essential? Maximize Blast Furnace Efficiency & Stability
- What are the advantages of using a customized multimode microwave reaction furnace? Boost Synthesis Speed by 90%
- What are some examples of low-temperature industrial heating processes? Boost Efficiency and Sustainability
- How does a high-temperature sintering furnace influence ZnO nanotube sensors? Unlock Peak Sensitivity and Stability



















