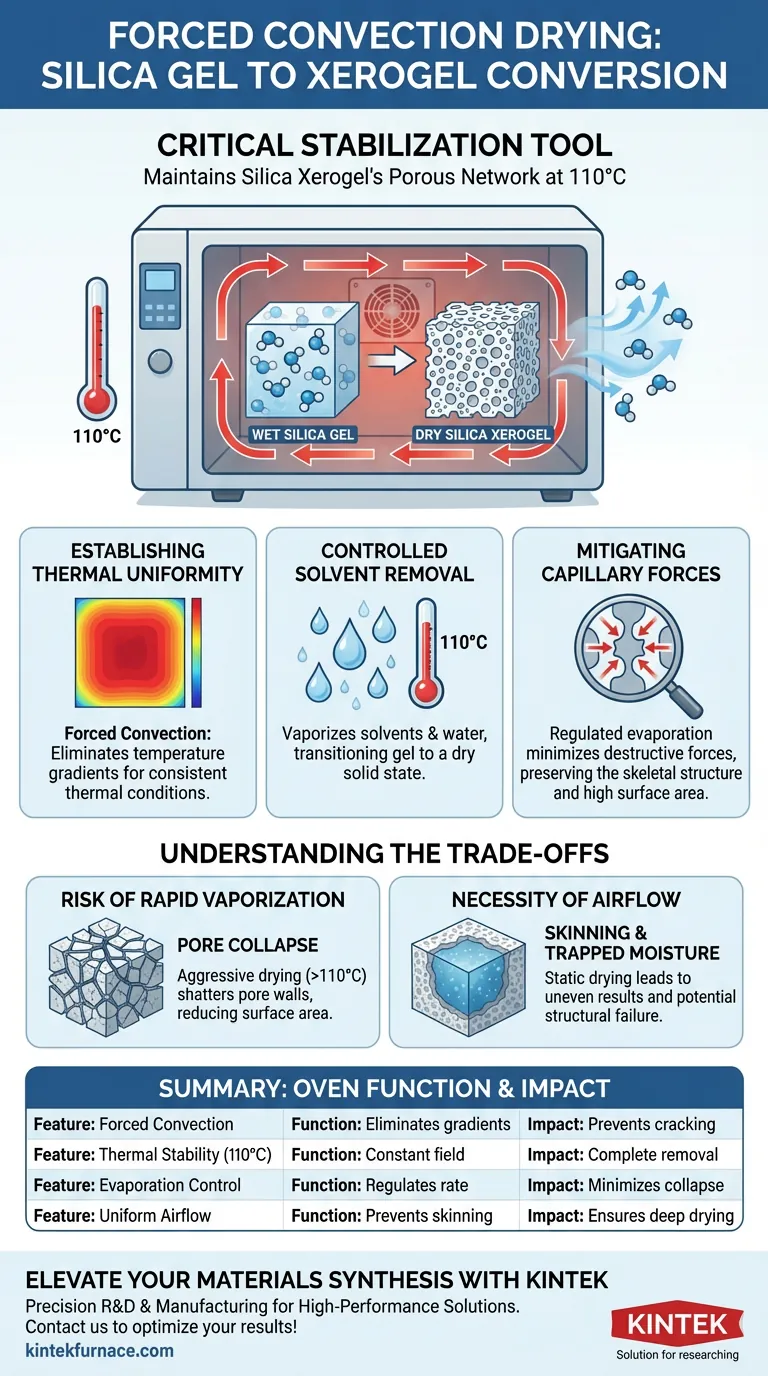
A forced convection drying oven serves as a critical stabilization tool during the synthesis of silica xerogel. Its primary function is to generate a consistent thermal field at 110 °C, which facilitates the controlled removal of solvents and moisture from the silica gel's porous network while preserving its delicate skeletal structure.
The oven acts as a safeguard against structural failure. By regulating the evaporation rate through uniform heating, it minimizes the destructive capillary forces that typically crush pore structures during drying, ensuring the final material retains a high specific surface area.

The Mechanics of Structural Preservation
Establishing Thermal Uniformity
The "forced convection" aspect of the oven is essential for process consistency. Unlike static ovens, it actively circulates air to eliminate temperature gradients.
This ensures that every part of the silica gel sample experiences the exact same thermal conditions. Uniform heat distribution prevents uneven drying rates, which could otherwise lead to internal stresses and cracking within the material.
Controlled Solvent Removal
Operating at 110 °C is a specific requirement for this conversion process. This temperature is sufficient to vaporize solvents and physically adsorbed water held within the gel’s pores.
It effectively transitions the material from a "wet" gel state to a dry solid state. This step is the defining moment where the substance officially converts from silica gel into silica xerogel.
Mitigating Capillary Forces
The most significant threat to silica xerogel quality is pore collapse. As liquid evaporates from the pores, surface tension creates powerful capillary forces that pull the pore walls inward.
The forced convection oven provides a steady, controlled evaporation environment. This prevents the rapid, violent vaporization that would exacerbate these forces, allowing the silica skeleton to withstand the drying process intact.
Understanding the Trade-offs
The Risk of Rapid Vaporization
While speed is often desired in manufacturing, drying silica gel too aggressively is detrimental. If the temperature exceeds the optimal range or fluctuates wildy, the liquid inside the pores vaporizes instantly.
This rapid expansion can shatter the microscopic pore walls. The result is a densified material with significantly reduced surface area, rendering the xerogel useless for applications requiring high porosity.
The Necessity of Airflow
Relying on standard conduction heating (without forced air) often results in "skinning," where the outer layer dries while the inside remains wet.
This traps moisture inside the gel. Trapped moisture can lead to structural failure during later stages or result in inaccurate quantitative analysis regarding the material's composition.
Making the Right Choice for Your Goal
To ensure the successful conversion of silica gel to high-quality xerogel, you must prioritize stability over speed.
- If your primary focus is High Specific Surface Area: Ensure the oven temperature is strictly maintained at 110 °C to balance solvent removal with skeletal preservation.
- If your primary focus is Structural Consistency: Verify that the forced convection mechanism is functioning correctly to prevent hot spots and ensure uniform drying across the entire sample batch.
Mastering this drying stage is the key to producing a robust xerogel with the maximum possible reactive surface area.
Summary Table:
| Feature | Function in Silica Xerogel Conversion | Impact on Material Quality |
|---|---|---|
| Forced Convection | Eliminates temperature gradients & hot spots | Prevents internal stress and cracking |
| Thermal Stability | Maintains a constant 110 °C field | Ensures complete solvent & moisture removal |
| Evaporation Control | Regulates the rate of liquid vaporization | Minimizes capillary forces to prevent pore collapse |
| Uniform Airflow | Prevents surface "skinning" | Ensures deep drying and structural consistency |
Elevate Your Materials Synthesis with KINTEK
Precision is the difference between a high-performance xerogel and a collapsed structure. Backed by expert R&D and manufacturing, KINTEK provides high-performance forced convection ovens and specialized lab high-temp furnaces—including Muffle, Tube, Rotary, and Vacuum systems—engineered to maintain the strict thermal uniformity your research demands.
Whether you need a standard solution or a system fully customizable for your unique drying protocols, our technical team is ready to help you optimize your results.
Ready to preserve your material's integrity? Contact us today to discuss your project requirements!
Visual Guide
References
- Raden Darmawan, Fitria Nur Laily. Silica Synthesis from Mount Semeru Volcanic Ash as a Nickel Heavy Metal Adsorbent. DOI: 10.9767/bcrec.20337
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is the objective of setting temperature gradients of 40 °C, 50 °C, and 60 °C? Optimize Yogurt Drying Viability
- How does a heated substrate platform mitigate the coffee ring effect? Enhance Ag2Se Printing Precision
- Why is a high-pressure stainless steel autoclave required for activated carbon? Unlock High-Performance Carbon Synthesis
- Why are advanced materials and composites important? Unlock Next-Gen Performance in Aerospace, Auto, and More
- What are the ideal characteristics of a quenching medium? Achieve Optimal Hardness and Safety in Heat Treatment
- How does microstructural observation assist in optimizing LATP sintering? Master High-Density Material Processing
- Why is a precision furnace required after TiO2-alpha-Ga2O3 synthesis? Master Phase Transformation & Interface Bonding
- What are the limitations of PVD coating? Overcome Challenges for Optimal Surface Engineering



















