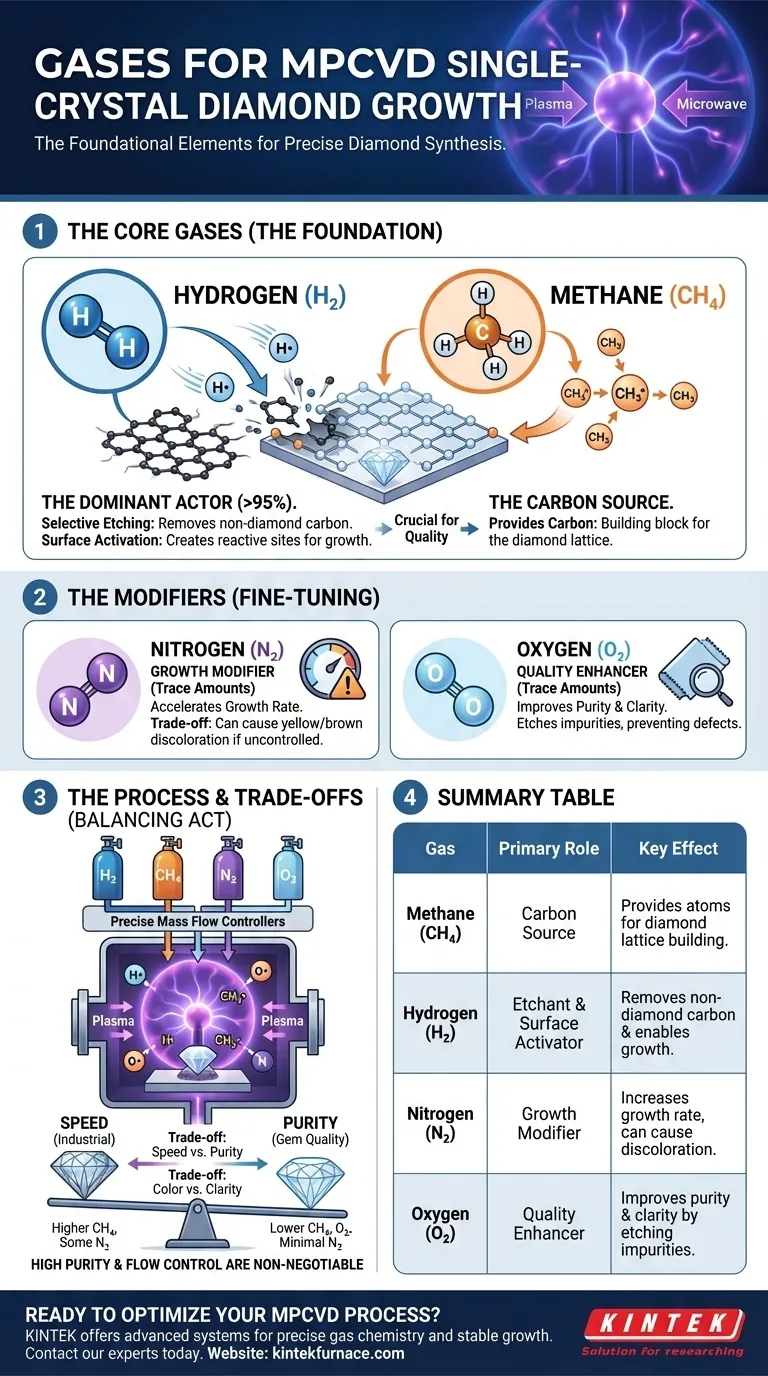
In MPCVD diamond synthesis, a precise mixture of specific gases is the foundational element of the entire process. The most common gases used are a carbon source, typically methane (CH4), and a vast excess of hydrogen (H2). To fine-tune the growth process and final crystal properties, small, controlled amounts of other gases like nitrogen (N2) and oxygen (O2) are also strategically introduced into the plasma.
While methane provides the carbon atoms to build the diamond, hydrogen is the true workhorse of the process. It selectively etches away undesirable non-diamond carbon and creates the active surfaces necessary for high-quality, single-crystal growth.

The Role of Each Gas in the Plasma Environment
In an MPCVD (Microwave Plasma Chemical Vapor Deposition) system, microwaves energize the gas mixture into a plasma—a highly reactive state of atomic and molecular fragments. Each gas plays a distinct and critical role in this environment.
The Carbon Source: Methane (CH4)
Methane is the primary provider of carbon, the building block of diamond.
The intense energy of the microwave plasma breaks down the stable CH4 molecules into reactive carbon-containing radicals, such as CH3. These fragments are the species that actually incorporate into the diamond lattice on the seed crystal.
The Dominant Actor: Hydrogen (H2)
Hydrogen typically makes up over 95% of the gas mixture and serves two essential functions.
First, atomic hydrogen (H) from the plasma performs selective etching. It aggressively removes any graphitic or amorphous (non-diamond) carbon that inadvertently forms on the growing surface. This purification step is crucial for achieving a pure, single-crystal structure.
Second, hydrogen activates the growth surface. It terminates the diamond surface, creating a stable platform. The atomic hydrogen can then abstract a surface hydrogen atom, creating a reactive "dangling bond" where a carbon radical (like CH3) can attach and continue the lattice growth.
The Growth Modifier: Nitrogen (N2)
Nitrogen is often added in very small, deliberate amounts (parts per million) to influence the growth characteristics.
Its primary effect is to accelerate the crystal growth rate, which is a significant commercial advantage. Nitrogen can promote the formation of specific growth sites on the crystal surface, speeding up carbon incorporation.
The Quality Enhancer: Oxygen (O2)
Trace amounts of oxygen can also be added to the gas mixture to improve the final quality and growth efficiency.
Like hydrogen, oxygen-containing species (such as O and OH radicals) are highly effective at etching away non-diamond carbon impurities. This can widen the range of conditions for stable growth and help produce clearer, more colorless diamonds by preventing defect formation.
Understanding the Trade-offs and Control
The success of MPCVD diamond growth hinges on precisely balancing the ratios of these gases, as each introduces a critical trade-off.
The Methane-to-Hydrogen Ratio
This ratio is the most fundamental control parameter. A higher methane concentration leads to faster growth but increases the risk of forming lower-quality polycrystalline diamond or graphite. A lower concentration produces higher-purity crystals but at a much slower, less economical rate.
The Double-Edged Sword of Nitrogen
While nitrogen boosts growth speed, it is also the most common impurity in diamond. If too much nitrogen gets incorporated into the crystal lattice, it imparts an undesirable yellow or brown color. Controlling its concentration is the key to balancing speed with gem quality.
The Importance of Purity and Flow
The entire process is acutely sensitive to contaminants. High-purity source gases and precise mass flow controllers are non-negotiable. The vacuum systems are not just for creating a low-pressure environment but for ensuring that no atmospheric leaks or impurities disrupt the delicate chemical balance inside the chamber.
Optimizing the Gas Mixture for Your Goal
The ideal gas composition is not a single formula but is tailored to the desired outcome of the diamond growth.
- If your primary focus is maximum growth speed for industrial applications: A higher methane concentration and a controlled addition of nitrogen are often employed to prioritize volume.
- If your primary focus is the highest purity and colorless gem quality: A lower methane-to-hydrogen ratio is used, often with a small amount of oxygen and minimal to no nitrogen, to prioritize perfection over speed.
- If your primary focus is creating specific colored diamonds (e.g., yellow): A deliberate and precisely managed amount of nitrogen is introduced into the gas flow throughout the growth cycle.
Ultimately, mastering MPCVD is a matter of mastering the complex chemistry of this reactive gas environment.
Summary Table:
| Gas | Primary Role | Key Effect on Growth |
|---|---|---|
| Methane (CH₄) | Carbon Source | Provides atoms for diamond lattice building. |
| Hydrogen (H₂) | Etchant & Surface Activator | Removes non-diamond carbon and enables growth. |
| Nitrogen (N₂) | Growth Modifier | Increases growth rate but can cause discoloration. |
| Oxygen (O₂) | Quality Enhancer | Improves purity and clarity by etching impurities. |
Ready to Optimize Your MPCVD Process?
Mastering the precise gas chemistry is the key to successful single-crystal diamond growth. The right MPCVD system provides the stable, controllable environment necessary for this delicate balance.
KINTEK's advanced MPCVD systems are engineered for this exact challenge. Leveraging our exceptional in-house R&D and manufacturing capabilities, we provide laboratories with robust solutions featuring:
- Precise Mass Flow Control for accurate gas mixture management.
- Stable Plasma Environments essential for consistent, high-quality growth.
- Deep Customization to tailor the system to your unique research or production goals, whether you prioritize speed, purity, or specific crystal properties.
Let's discuss how we can help you achieve your diamond synthesis objectives.
Contact our experts today to explore the ideal MPCVD solution for your lab.
Visual Guide
Related Products
- Cylindrical Resonator MPCVD Machine System for Lab Diamond Growth
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- HFCVD Machine System Equipment for Drawing Die Nano Diamond Coating
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
People Also Ask
- Why is temperature control important in the MPCVD growth process? Ensure High-Quality, Reliable Diamond Film Deposition
- How does the MPCVD deposition process work? Unlock Superior Diamond Film Quality
- How does MPCVD compare to other CVD techniques like HFCVD and PECVD? Discover the Best for High-Purity Films
- What are the key features of MPCVD single crystal diamond deposition equipment? Precision Control for High-Quality Growth
- What factors influence the quality of diamond deposition in the MPCVD method? Master the Critical Parameters for High-Quality Diamond Growth
- What are the key advantages of MPCVD in diamond synthesis? Achieve High-Purity, Scalable Diamond Production
- What effect does the sample base position have in an MPCVD device? Master Plasma Control for Optimal Deposition
- What challenges does MPCVD face despite its advantages? Balancing Growth Rate, Quality, and Scalability



















