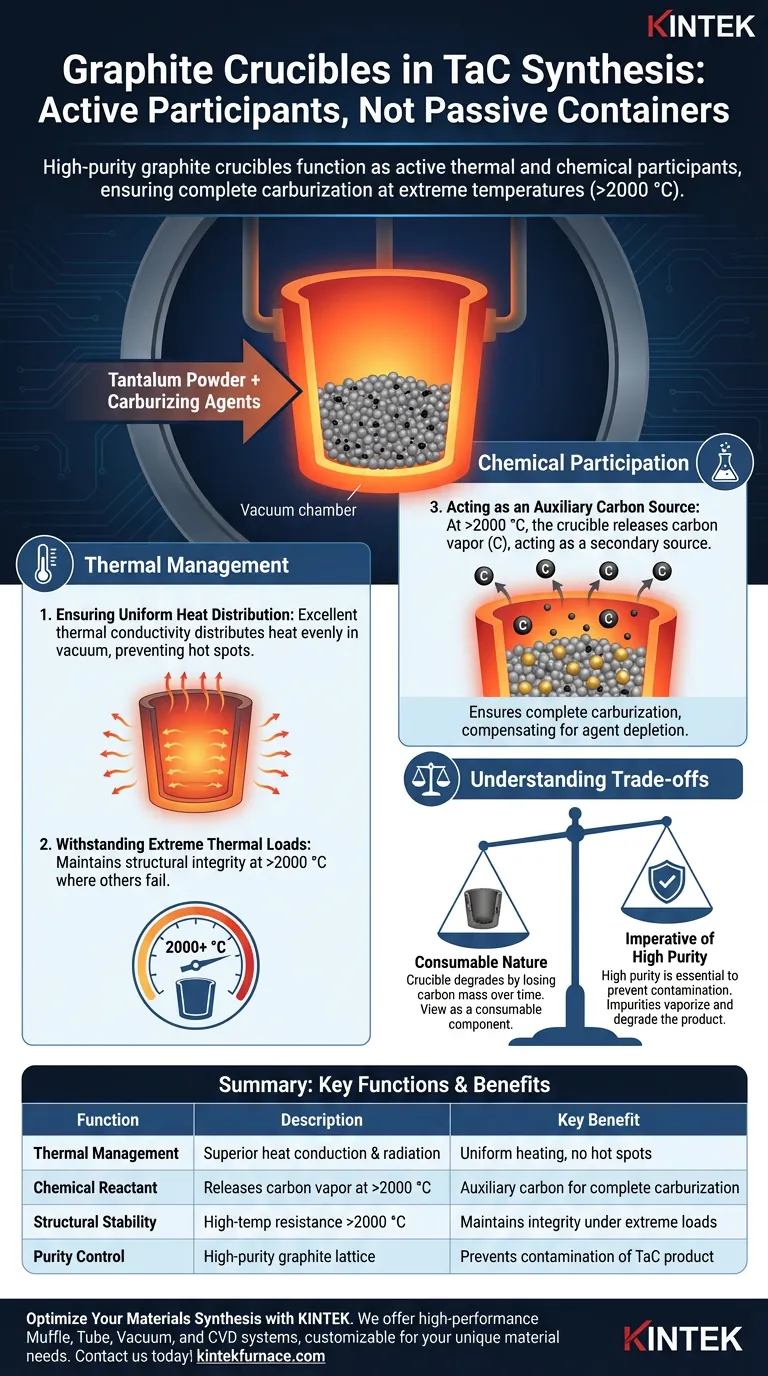
High-purity graphite crucibles function as active thermal and chemical participants in the synthesis process, not merely passive containers. In the vacuum synthesis of tantalum carbide, they serve to securely hold the tantalum powder and carburizing agents while leveraging exceptional thermal conductivity to ensure uniform heating. Crucially, at temperatures exceeding 2000 °C, the crucible itself acts as a supplementary reactant, releasing carbon vapor to aid the carburization process.
Core Takeaway While most crucibles are designed solely to isolate samples, high-purity graphite is selected for its ability to integrate into the reaction kinetics. Its capacity to act as an auxiliary carbon source at extreme temperatures ensures complete carburization, making it indispensable for synthesizing high-quality tantalum carbide.

The Role of Thermal Management
Ensuring Uniform Heat Distribution
In vacuum environments, heat transfer relies heavily on radiation and conduction rather than convection.
High-purity graphite possesses excellent thermal conductivity properties. This allows the crucible to distribute heat evenly across the tantalum powder mixture, preventing hot spots or cold zones that could lead to inconsistent material properties.
Withstanding Extreme Thermal Loads
The synthesis of tantalum carbide requires processing temperatures that can exceed 2000 °C.
Graphite exhibits exceptional high-temperature resistance, maintaining structural integrity where many other materials would melt or fracture. This stability ensures the vessel remains intact throughout the intense heating cycle required to drive the reaction.
Chemical Participation in Synthesis
Acting as an Auxiliary Carbon Source
Perhaps the most distinct function of the graphite crucible in this context is its chemical contribution.
At temperatures surpassing 2000 °C, the graphite material begins to release trace amounts of carbon vapor. This vapor permeates the reaction zone, acting as a secondary source of carbon.
This ensures that the tantalum powder is fully carburized, effectively compensating for any potential depletion of the primary carburizing agents in the mixture.
Understanding the Trade-offs
The Consumable Nature of the Crucible
Because the crucible participates in the reaction by losing carbon mass, it inevitably degrades over time.
Users must view these crucibles as consumable components rather than permanent fixtures. The "auxiliary carbon source" feature means the crucible walls will thin after repeated cycles at extreme temperatures.
The Imperative of High Purity
The "high-purity" specification is not a marketing term; it is a chemical necessity.
Any impurities present in the graphite lattice will vaporize along with the carbon at high temperatures. These impurities can contaminate the tantalum carbide, degrading its mechanical or thermal properties. Therefore, strict adherence to purity standards is required to prevent cross-contamination.
Making the Right Choice for Your Goal
When designing your synthesis process, consider how the crucible interacts with your specific parameters:
- If your primary focus is stoichiometric precision: Account for the extra carbon provided by the crucible vapor at >2000 °C to prevent over-carburization or to balance the reactant mix.
- If your primary focus is material purity: Verify the specific impurity trace analysis of the graphite grade, as any non-carbon elements in the crucible will likely migrate into your final product.
Success in tantalum carbide synthesis depends on treating the crucible as a dynamic variable in your chemical equation.
Summary Table:
| Function | Description | Key Benefit |
|---|---|---|
| Thermal Management | Superior heat conduction and radiation in vacuum. | Ensures uniform heating and prevents hot spots. |
| Chemical Reactant | Releases carbon vapor at temperatures >2000 °C. | Acts as an auxiliary carbon source for complete carburization. |
| Structural Stability | High-temperature resistance exceeding 2000 °C. | Maintains integrity under extreme thermal loads. |
| Purity Control | Use of high-purity graphite lattice. | Prevents contamination of the tantalum carbide product. |
Optimize Your Materials Synthesis with KINTEK
Precision in high-temperature synthesis requires more than just high-quality components; it requires the right equipment environment. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized lab high-temp furnaces—all customizable for your unique material needs.
Whether you are synthesizing advanced carbides or perfecting thermal cycles, our team provides the technical expertise and robust systems to ensure your success. Contact us today to find the perfect high-temperature solution for your lab!
Visual Guide
References
- Seon-Min Hwang, Dong‐Won Lee. Carburization of Tantalum Metal Powder Using Activated Carbon. DOI: 10.3390/ma18122710
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Magnesium Extraction and Purification Condensing Tube Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What factors influence the lifespan of alumina ceramic furnace tubes? Maximize Durability and Performance
- Why is a gas mixing system essential for syngas annealing in copper powder production? Ensure Precise Embrittlement
- Why are metal wire mesh trays preferred for thin-layer drying? Boost Efficiency and Accuracy in Your Lab
- What is the function of a honeycomb-shaped firing tray? Master Thermal Equilibrium in Ceramic Sintering
- What role does a water saturator play in the physical activation of carbon materials? Unlock High-Performance Porosity
- What role do substrate heaters play in Ga2O3:Er thin films? Unlock Crystalline Beta-Phase Transitions
- What role does a laboratory vacuum pump play in a static batch desulfurization evaluation system? Ensure Data Integrity
- Why is a molybdenum crucible considered an ideal choice for quartz melting? High-Purity Solutions at 2000°C



















