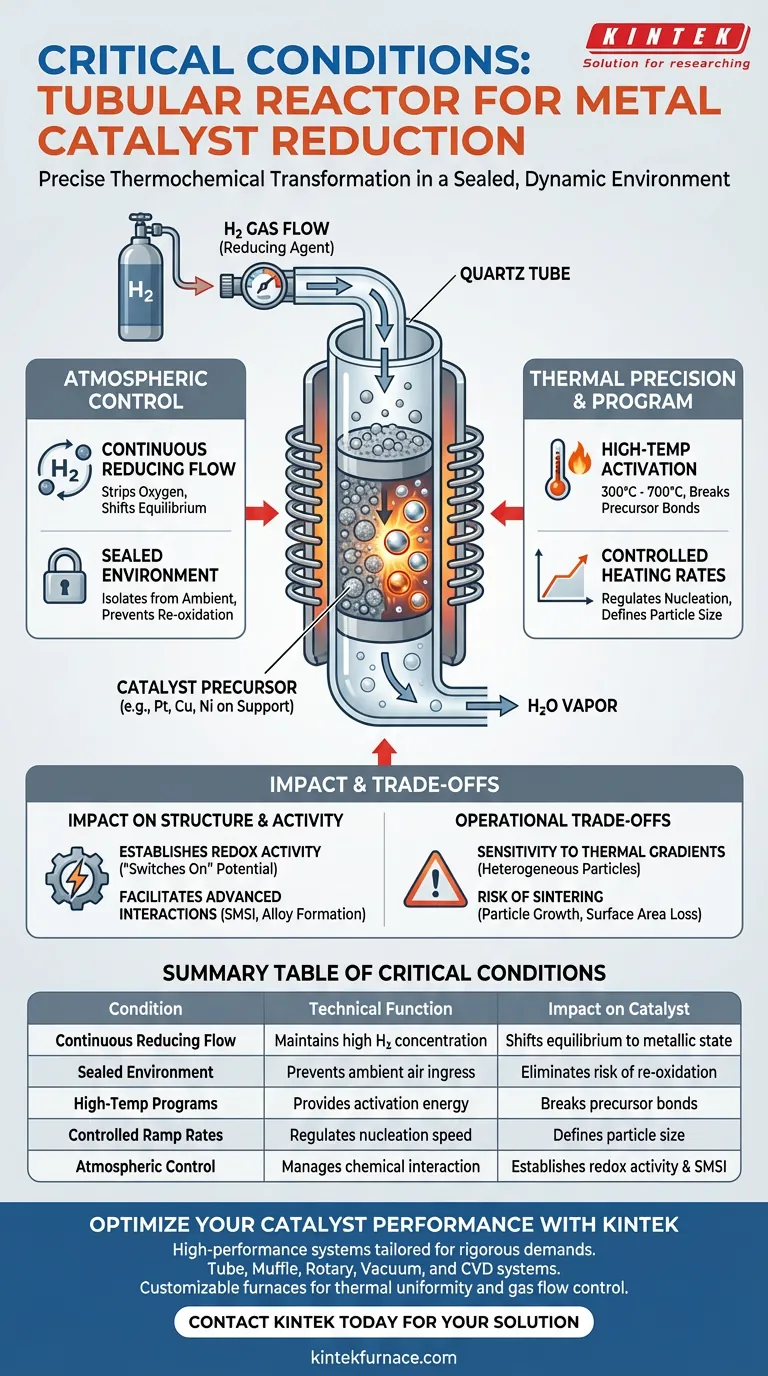
A tubular reactor provides a sealed, dynamic environment essential for the precise thermochemical transformation of metal catalysts. By maintaining a continuous flow of reducing gas—typically hydrogen—and executing specific high-temperature programs, it ensures that inactive metal precursors are effectively converted into their active metallic states.
Catalyst reduction is not merely about heating; it is about synchronizing chemical reduction with structural formation. The tubular reactor facilitates this by strictly governing the interaction between thermal energy and the reducing atmosphere, granting catalysts like platinum, copper, and nickel their required redox activity.
The Critical Role of Atmospheric Control
Continuous Reducing Flow
The primary function of the tubular reactor is to maintain a continuous flow of hydrogen gas (often mixed with inert gases like Argon).
This flow is critical for stripping oxygen from metal precursors. It ensures that the chemical equilibrium constantly shifts toward the metallic elemental state.
A Sealed Environment
The reactor creates a hermetically sealed system that isolates the catalyst from the ambient environment.
This isolation prevents the re-oxidation of sensitive metals during the critical transition phase. It guarantees that the reduction process is driven solely by the introduced gas stream, ensuring high purity.
Thermal Precision and Program Execution
High-Temperature Activation
Reduction requires significant thermal energy to break precursor bonds. The tubular reactor executes specific high-temperature programs, often reaching 300°C for standard reductions or up to 700°C for advanced applications.
This thermal energy is the driving force that converts precursors on carbon supports into their final metallic forms.
Controlled Heating Rates
Beyond just reaching a target temperature, the reactor allows for precise control of the heating rate.
Regulating how fast the temperature rises is essential for controlling the initial nucleation of metal particles. This precision helps define the final particle size and prevents uncontrolled aggregation.
Impact on Catalyst Structure and Activity
Establishing Redox Activity
The ultimate goal of these conditions is to grant the catalyst its redox activity.
By effectively reducing precursors to their metallic states, the reactor "switches on" the chemical potential of metals like platinum and nickel, making them ready for reaction.
Facilitating Advanced Interactions
In more complex syntheses, such as NiCu alloys or cobalt systems, the reactor's conditions promote Strong Metal-Support Interaction (SMSI).
The combination of high heat and reducing atmosphere can drive the migration of support layers onto the metal surface. This creates encapsulation shells or induces tensile strain, which can tune the electronic properties of the catalyst.
Understanding the Operational Trade-offs
Sensitivity to Thermal Gradients
While tubular reactors offer precision, they require careful management of thermal uniformity across the tube length.
If the temperature profile is not uniform, different sections of the catalyst bed may reduce at different rates. This can lead to heterogeneous particle sizes, reducing the overall efficiency of the batch.
The Risk of Sintering
The same high temperatures required for reduction can inadvertently cause sintering (the merging of small particles into larger, less active ones).
If the heating ramp is too aggressive or the hold time too long, the surface area of the catalyst may decrease. Balancing complete reduction against particle growth is the central challenge of the process.
Making the Right Choice for Your Synthesis
To maximize the potential of your catalyst, align your reactor settings with your specific material goals:
- If your primary focus is basic activation: Prioritize a steady hydrogen flow and a moderate temperature program (around 300°C) to ensure complete conversion to the metallic state.
- If your primary focus is alloy formation or SMSI: Utilize higher temperatures (up to 700°C) and precise ramp rates to drive atomic migration and structural strain without causing excessive sintering.
The quality of your final catalyst is a direct reflection of the precision with which you control its reduction environment.
Summary Table:
| Critical Condition | Technical Function | Impact on Catalyst |
|---|---|---|
| Continuous Reducing Flow | Maintains high H2 concentration | Shifts equilibrium to metallic state |
| Sealed Environment | Prevents ambient air ingress | Eliminates risk of re-oxidation |
| High-Temp Programs | Provides activation energy | Breaks precursor bonds for conversion |
| Controlled Ramp Rates | Regulates nucleation speed | Defines particle size and prevents aggregation |
| Atmospheric Control | Manages chemical interaction | Establishes redox activity and SMSI |
Optimize Your Catalyst Performance with KINTEK
Precision in thermal processing is the difference between a high-activity catalyst and a failed batch. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems tailored for the rigorous demands of metal catalyst reduction.
Our customizable high-temperature furnaces provide the thermal uniformity and gas flow control required for delicate platinum, copper, and nickel syntheses. Whether you are scaling up production or refining atomic-scale interactions, our engineering team ensures your lab has the exact tools needed for success.
Ready to elevate your material synthesis? Contact KINTEK today to find the perfect furnace solution for your unique needs.
Visual Guide
References
- Lucien Magson, Diego Sampedro. Synthesis and Characterization of Carbon-Based Heterogeneous Catalysts for Energy Release of Molecular Solar Thermal Energy Storage Materials. DOI: 10.1021/acsami.3c16855
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What safety features are typically included in vacuum tube furnaces? Essential Protection for High-Temp Labs
- How does a dual-temperature zone tube furnace contribute to the carbonization of biomass? Precise Material Engineering
- What role does a continuous bench-scale drop tube pyrolyzer play in FPBO? Maximize High-Quality Bio-oil Yields
- What is the technical value of using an industrial-grade tube furnace for titania nanotubes? Enhance Crystal Performance
- What is the function of an industrial-grade tube furnace? Mastering Expanded Graphite (EG) Calcination
- What materials are used for the tubes in drop tube furnaces and why? Choose the Right Tube for High-Temp Success
- How does a high-temperature tube furnace form Nitrogen-doped Porous Carbon (RMF)? Precision Thermal Synthesis Guide
- How does secondary pyrolysis activation in a tube furnace at 800 °C contribute to the pore structure of APC?



















