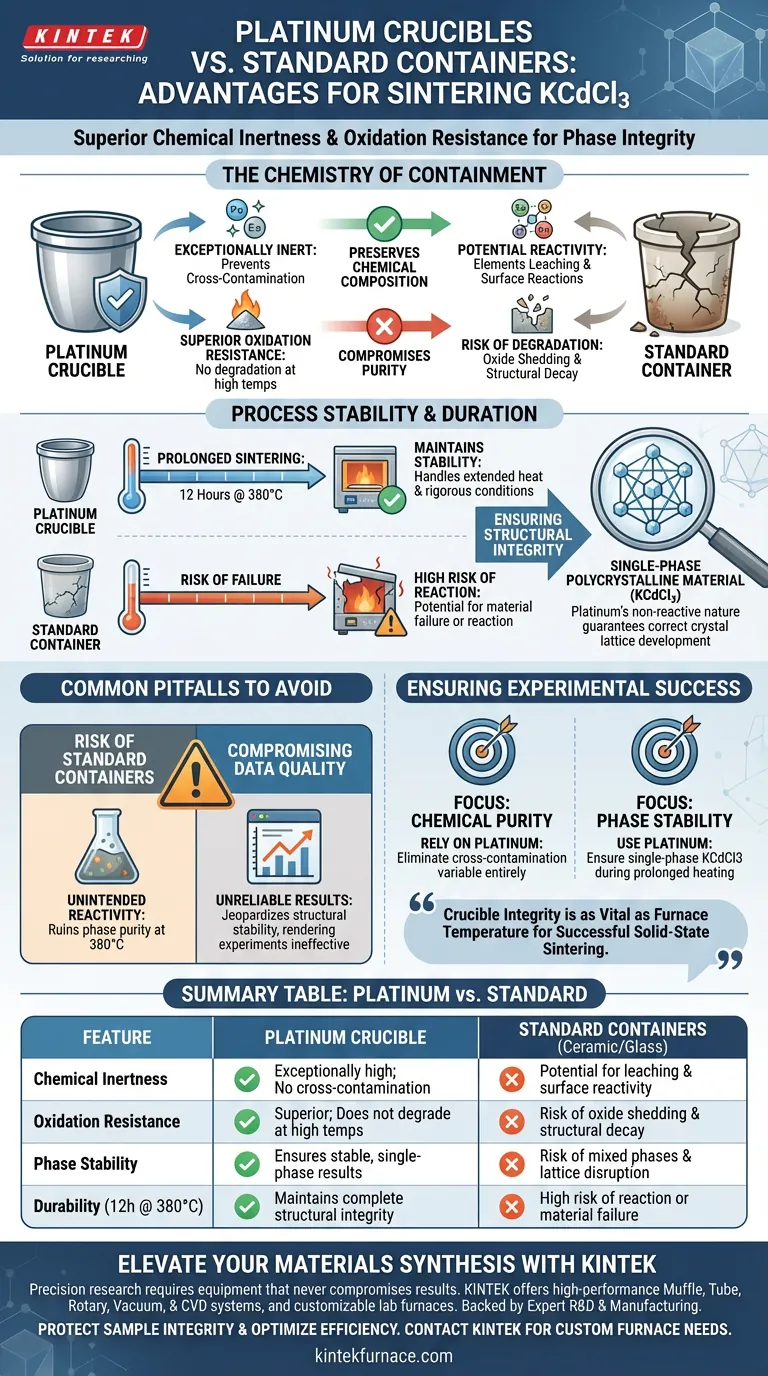
Platinum crucibles provide a critical advantage in the sintering of KCdCl3 by offering superior chemical inertness and oxidation resistance. Unlike standard containers, platinum prevents cross-contamination during the heating process, ensuring that the synthesized material retains its intended purity and structural composition.
The core value of using platinum is the preservation of phase integrity. By eliminating the risk of reaction between the vessel and the sample, platinum ensures the final KCdCl3 remains a stable, single-phase polycrystalline material.

The Chemistry of Containment
Preventing Cross-Contamination
When sintering halide perovskites like KCdCl3, the choice of container is not merely a matter of holding the powder. Standard containers often possess surface reactivities that can lead to elements leaching into the sample.
Platinum provides an exceptionally inert environment. This inertness acts as a barrier, ensuring that the chemical composition of the sample is not altered by the vessel itself.
Withstanding Oxidation
Solid-state sintering is a rigorous process involving elevated temperatures and specific atmospheric conditions. Platinum is highly resistant to oxidation, meaning the crucible itself does not degrade or shed oxide particles into the sample.
This is particularly important given the sensitivity of KCdCl3 to impurities. A degrading vessel would introduce foreign contaminants that compromise the experiment.
Process Stability and Duration
Handling Prolonged Sintering
The synthesis of high-quality KCdCl3 often requires extended exposure to heat to achieve the correct crystalline structure.
Specifically, the process may involve sintering for durations such as 12 hours at 380 degrees Celsius. Platinum maintains its stability throughout these long cycles, where lesser materials might fail or react.
Ensuring Structural Integrity
The ultimate goal of this process is to produce single-phase polycrystalline particles.
If the container reacts with the sample, it can disrupt the crystal lattice, leading to mixed phases or structural instability. Platinum’s non-reactive nature guarantees that the physical structure of the KCdCl3 develops exactly as intended.
Common Pitfalls to Avoid
The Risk of Standard Containers
While standard ceramic or glass containers are common in general chemistry, they introduce significant risks in this specific application.
The primary pitfall is unintended reactivity. At 380 degrees Celsius, standard materials may facilitate ion exchange or surface reactions that ruin the phase purity of the perovskite.
Compromising Data Quality
If the vessel alters the sample, any subsequent data regarding the material's properties becomes unreliable.
Using a reactive crucible is a false economy; it jeopardizes the structural stability of the final product, rendering the sintering process ineffective for high-precision applications.
Ensuring Experimental Success
To achieve high-quality synthesis results, align your equipment choice with your specific scientific goals.
- If your primary focus is Chemical Purity: Rely on platinum crucibles to eliminate the variable of cross-contamination entirely.
- If your primary focus is Phase Stability: Use platinum to ensure the KCdCl3 remains single-phase during prolonged heating at 380°C.
The integrity of your crucible is just as vital as the accuracy of your furnace temperature for successful solid-state sintering.
Summary Table:
| Feature | Platinum Crucible | Standard Containers (Ceramic/Glass) |
|---|---|---|
| Chemical Inertness | Exceptionally high; no cross-contamination | Potential for leaching and surface reactivity |
| Oxidation Resistance | Superior; does not degrade at high temps | Risk of oxide shedding and structural decay |
| Phase Stability | Ensures stable, single-phase results | Risk of mixed phases and lattice disruption |
| Durability (12h @ 380°C) | Maintains complete structural integrity | High risk of reaction or material failure |
Elevate Your Materials Synthesis with KINTEK
Precision research requires equipment that never compromises your results. KINTEK provides high-performance solutions designed for the most demanding solid-state sintering processes. Backed by expert R&D and manufacturing, we offer a full range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable lab high-temperature furnaces tailored to your unique specifications.
Whether you are sintering halide perovskites or developing advanced polycrystalline materials, our systems ensure the thermal stability and accuracy you need. Protect your sample integrity and optimize your lab's efficiency—Contact KINTEK today to discuss your custom furnace needs!
Visual Guide
References
- Md. Sunjid Sorker, Md. Abdur Razzak Sarker. First-principles and experimental study to investigate structural, elastic, electronic, thermal, and optical properties of KCdCl3 metal halide perovskite crystals. DOI: 10.1063/5.0206191
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the primary function of a drying oven during LLZTO preparation? Ensure Pure Phase Solid Electrolytes
- What role does specialized graphite adhesive play? Expert Bonding Solutions for High-Temp Systems
- What are the advantages of nickel crucibles for KOH activation? Ensure High Purity & Thermal Stability up to 700°C
- How does the use of Matched Thermal Baffles (MTB) benefit directional solidification? Achieve Superior Crystal Integrity
- What are the technical advantages of using quartz tubes for fiber optic sensors? Optimize High-Temp Performance
- Why are high-purity alumina crucibles selected for lithium orthosilicate synthesis? Ensure Purity & Thermal Stability
- What role do substrate heaters play in Ga2O3:Er thin films? Unlock Crystalline Beta-Phase Transitions
- How does a laboratory blast drying oven facilitate the conversion of acid leach liquor into solid PAFS? Key Mechanism



















