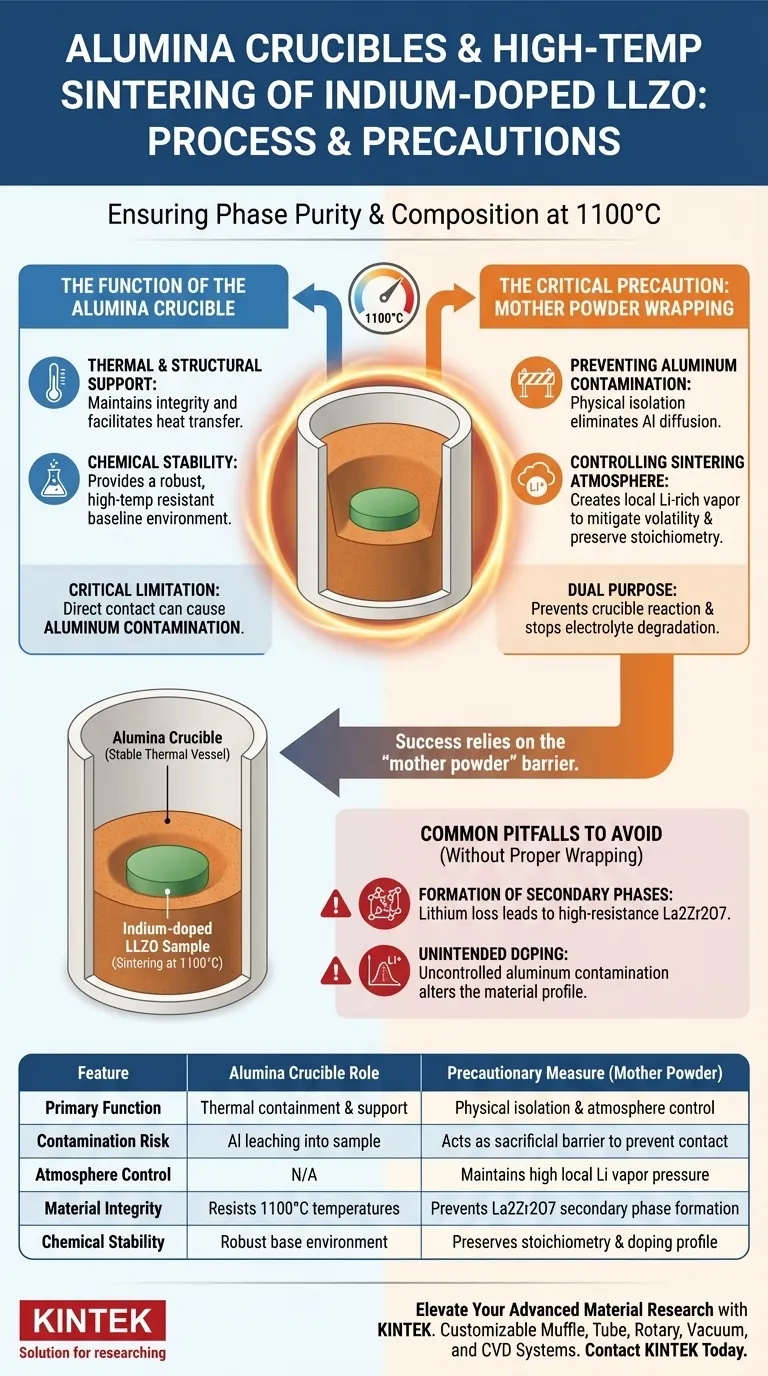
In the high-temperature sintering of Indium-doped LLZO, an alumina crucible functions as a stable thermal vessel to support the sample and facilitate heat transfer at 1100°C. However, simply placing the sample inside is insufficient; a critical mother powder wrapping technique is employed to cover the sample with loose powder of the same composition, physically isolating it from the crucible walls to prevent aluminum contamination.
While the alumina crucible provides the necessary high-temperature resistance, the success of the process relies on the "mother powder" barrier. This technique solves two problems simultaneously: it prevents reactive impurities from leaching out of the crucible and creates a lithium-rich local atmosphere to stop the electrolyte from degrading.

The Function of the Alumina Crucible
Thermal and Structural Support
At sintering temperatures of 1100°C, the alumina crucible serves as the primary containment vessel. Its high thermal stability allows it to maintain structural integrity while effectively transferring heat to the Indium-doped LLZO sample.
Chemical Stability
Alumina is selected for its general high-temperature chemical resistance. It provides a robust baseline environment intended to prevent external impurities from entering the reaction zone.
The Critical Precaution: Mother Powder Wrapping
Preventing Aluminum Contamination
Despite the stability of alumina, direct contact between the crucible and the Indium-doped LLZO can lead to a chemical reaction. To mitigate this, the sample is wrapped or buried in "mother powder"—loose powder identical in composition to the sample.
Physical Isolation
This powder acts as a sacrificial physical barrier. It ensures the solid pellet never touches the alumina walls, effectively eliminating the risk of aluminum diffusing into the LLZO structure.
Controlling the Sintering Atmosphere
Mitigating Lithium Volatility
High temperatures usually cause lithium to volatilize, leading to material degradation. The mother powder creates a local equilibrium pressure of lithium vapor immediately surrounding the sample.
Preserving Stoichiometry
By maintaining this lithium-rich micro-environment, the technique suppresses the evaporation of lithium from the pellet. This prevents stoichiometric imbalance, ensuring the final material retains the correct chemical ratios.
Common Pitfalls to Avoid
Formation of Secondary Phases
If the protective powder barrier is insufficient, lithium loss will occur. This deficiency leads to the formation of undesirable secondary phases, most notably La2Zr2O7, which creates high resistance and degrades performance.
Unintended Doping
Failing to fully isolate the sample from the crucible results in aluminum leaching. While aluminum is sometimes used as a dopant, uncontrolled contamination from the crucible alters the intended doping profile of the Indium-doped material.
Making the Right Choice for Your Project
To ensure high-quality synthesis of Indium-doped LLZO, prioritize the setup of your sintering environment:
- If your primary focus is Phase Purity: Ensure the mother powder completely surrounds the sample to maintain stoichiometry and prevent the formation of La2Zr2O7.
- If your primary focus is Composition Control: Verify that the physical isolation from the alumina is absolute to prevent unintended aluminum contamination.
Success in this process is defined not just by the temperature reached, but by the integrity of the protective micro-environment created around the sample.
Summary Table:
| Feature | Alumina Crucible Role | Precautionary Measure (Mother Powder) |
|---|---|---|
| Primary Function | Thermal containment and structural support | Physical isolation and atmosphere control |
| Contamination Risk | Aluminum leaching into the LLZO sample | Acts as a sacrificial barrier to prevent contact |
| Atmosphere Control | N/A | Maintains high local lithium vapor pressure |
| Material Integrity | Resists 1100°C temperatures | Prevents La2Zr2O7 secondary phase formation |
| Chemical Stability | Robust base environment | Preserves stoichiometry and doping profile |
Elevate Your Advanced Material Research with KINTEK
Precise sintering requires more than just high temperatures; it demands the right environment. Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized lab high-temp furnaces—all fully customizable to meet your unique sintering requirements.
Whether you are synthesizing Indium-doped LLZO or developing next-gen battery materials, our equipment ensures the thermal stability and atmosphere control your project deserves. Contact KINTEK today to discuss your specific needs and discover how our high-performance solutions can enhance your laboratory's efficiency and results.
Visual Guide
References
- Alaa Alsawaf, Miriam Botros. Influence of In‐Doping on the Structure and Electrochemical Performance of Compositionally Complex Garnet‐Type Solid Electrolytes. DOI: 10.1002/sstr.202400643
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Why are laboratory precision stirrers and heating devices essential for synthesizing magnetic precursor solutions?
- What are the common uses for Alumina ceramic tubes? Ideal for High-Temp, Insulation, and Corrosion Resistance
- Why is an external cooling system vital for high-temperature furnace stability? Protect Your Research Integrity
- How do alumina ceramic tubes compare to quartz ceramic tubes in terms of thermal properties? Choose the Right Tube for High-Temp Success
- What are the benefits of sealing SAC305 solder in vacuum quartz tubes? Ensure High-Reliability Alloy Integrity
- What is the role of an infrared pyrometer in wood carbonization? Optimize Your High-Temp Thermal Control
- What is the primary function of high-purity graphite crucibles? Ensure Superior Purity in Aluminum Alloy Melting
- What is the purpose of a laboratory vacuum chamber in sacrificial material ink prep? Ensure Structural Integrity.



















