Precise temperature regulation is the single most critical variable determining the polymorphic outcome when synthesizing copper(II) orthoperiodate salts. The specific temperature setting of your laboratory furnace dictates whether you produce the stable blue alpha-phase or the metastable violet beta-phase. Without exact thermal control, you cannot selectively target these phases or ensure the purity required for single-crystal analysis.
Temperature acts as the definitive switch for polymorphic selection in this synthesis. Maintaining a specific setpoint determines the phase, while the precision of the cooling rate governs the quality and kinetics of crystal growth.

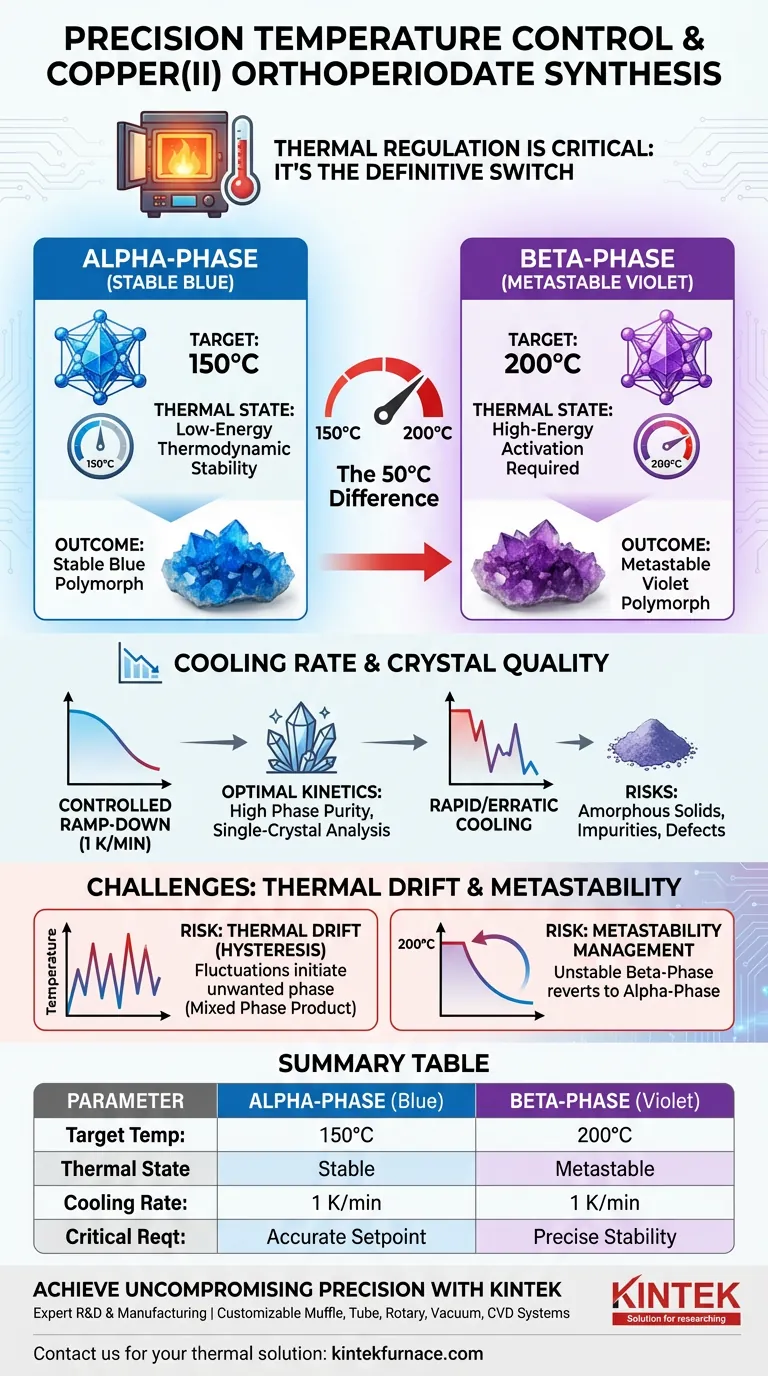
The Role of Temperature in Polymorphic Selection
The synthesis of copper(II) orthoperiodate is highly sensitive to thermal energy. The difference between creating one distinct chemical structure and another lies in a temperature shift of only 50 degrees Celsius.
Targeting the Stable Alpha-Phase
To synthesize the stable blue alpha-phase, you must maintain a reaction temperature of 150 degrees Celsius.
At this energy level, the thermodynamic conditions favor the formation of the alpha-polymorph. Stability is the defining characteristic here; this phase is the natural product of lower-energy thermal environments in this system.
Inducing the Metastable Beta-Phase
If you increase the furnace temperature to 200 degrees Celsius, the reaction pathway shifts.
This higher thermal energy induces the formation of the metastable violet beta-phase. Unlike the alpha-phase, this form relies on the elevated temperature to overcome the activation energy barrier required for its specific crystal lattice construction.
Beyond the Setpoint: The Importance of Cooling Rates
Setting the target temperature is only half of the precision equation. How the muffle furnace returns to ambient temperature is equally vital for the physical quality of the sample.
Controlling Crystal Growth Kinetics
The primary reference highlights a specific cooling rate of 1 K per minute.
This slow, controlled ramp-down prevents thermal shock and allows the crystal lattice to organize systematically. Rapid cooling often results in amorphous solids or micro-crystalline powders rather than distinct, usable crystals.
Achieving Phase Purity
Precise cooling is fundamental to maintaining high phase purity.
If the temperature drops too quickly or fluctuates during the cooling process, you risk trapping impurities or inducing defects in the crystal structure. A linear, controlled cooling rate ensures that the crystals grow with the structural integrity necessary for single-crystal analysis.
Understanding the Trade-offs
While high-temperature ovens and muffle furnaces are powerful tools, they present specific challenges in this synthesis that can compromise your results.
The Risk of Thermal Drift
Inexpensive or poorly calibrated furnaces often suffer from significant temperature fluctuation (hysteresis).
If you are targeting the alpha-phase at 150°C but your oven spikes to 180°C or higher due to poor control, you may inadvertently initiate the formation of the beta-phase. This results in a mixed-phase product that is chemically impure and unsuitable for characterization.
Metastability Management
The beta-phase is metastable, meaning it is energetically less stable than the alpha-phase.
If the temperature at 200°C is not maintained precisely, or if the cooling profile is erratic, the system may revert to the more stable alpha-phase. Precision is required not just to form the beta-phase, but to prevent it from degrading back into the alpha-phase during synthesis.
Making the Right Choice for Your Goal
To ensure experimental success, match your equipment settings to your specific synthetic targets.
- If your primary focus is the stable blue alpha-phase: Set your furnace strictly to 150°C to ensure thermodynamic stability.
- If your primary focus is the metastable violet beta-phase: Elevate the temperature to 200°C to access this higher-energy polymorph.
- If your primary focus is high-quality single crystals: Program a cooling ramp of exactly 1 K per minute to optimize growth kinetics.
Mastering the thermal profile of your furnace is the only way to guarantee the phase purity of your copper(II) orthoperiodate salts.
Summary Table:
| Parameter | Alpha-Phase (Stable Blue) | Beta-Phase (Metastable Violet) |
|---|---|---|
| Target Temperature | 150°C | 200°C |
| Thermal State | Low-energy thermodynamic stability | High-energy activation required |
| Cooling Rate | 1 K/min (Optimal for crystals) | 1 K/min (To prevent degradation) |
| Critical Requirement | Accurate setpoint to avoid drift | Precise stability to prevent reversion |
Achieve Uncompromising Thermal Precision with KINTEK
Precise temperature regulation is the difference between success and failure in advanced material synthesis. At KINTEK, we understand that even a 1 K/min cooling rate or a 50°C shift can redefine your experimental outcomes.
Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to eliminate thermal drift and ensure phase purity. Whether you are synthesizing copper(II) orthoperiodate salts or developing new metastable phases, our lab high-temperature furnaces are fully customizable to meet your unique research needs.
Ready to elevate your lab's synthesis capabilities? Contact us today to find the perfect thermal solution for your materials.
Visual Guide
References
- Two Polymorphs of the Magnetic <i>Catena</i> ‐Orthoperiodato‐Cuprate(II) K <sub>3</sub> [CuIO <sub>6</sub> ]·4H <sub>2</sub> O from Ultra‐Alkaline Media. DOI: 10.1002/zaac.202500092
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- Why must ceramic shells undergo high-temperature sintering? Ensure Integrity for High-Melting-Point Alloys
- What are the common heating elements used in muffle furnaces and their corresponding temperature ranges? Choose the Right Element for Your Lab
- What is the purpose of a muffle furnace in microbiological analysis? Achieve Absolute Sterility and Precise Sample Preparation
- What makes box furnaces suitable for demanding applications? Engineered for Precision and Durability in High-Stakes Processes
- What are the specific uses of muffle furnaces in laboratories? Essential for Contaminant-Free High-Temp Processes
- How are a muffle furnace and ceramic crucible used for MoO3? Master High-Purity Synthesis Today
- What is the purpose of using a furnace for BSCF sintering at 1000 °C? Engineer Superior Perovskite Structures
- What is the key role of a muffle furnace in the pretreatment of boron sludge and szaibelyite? Unlock Higher Process Efficiency



















