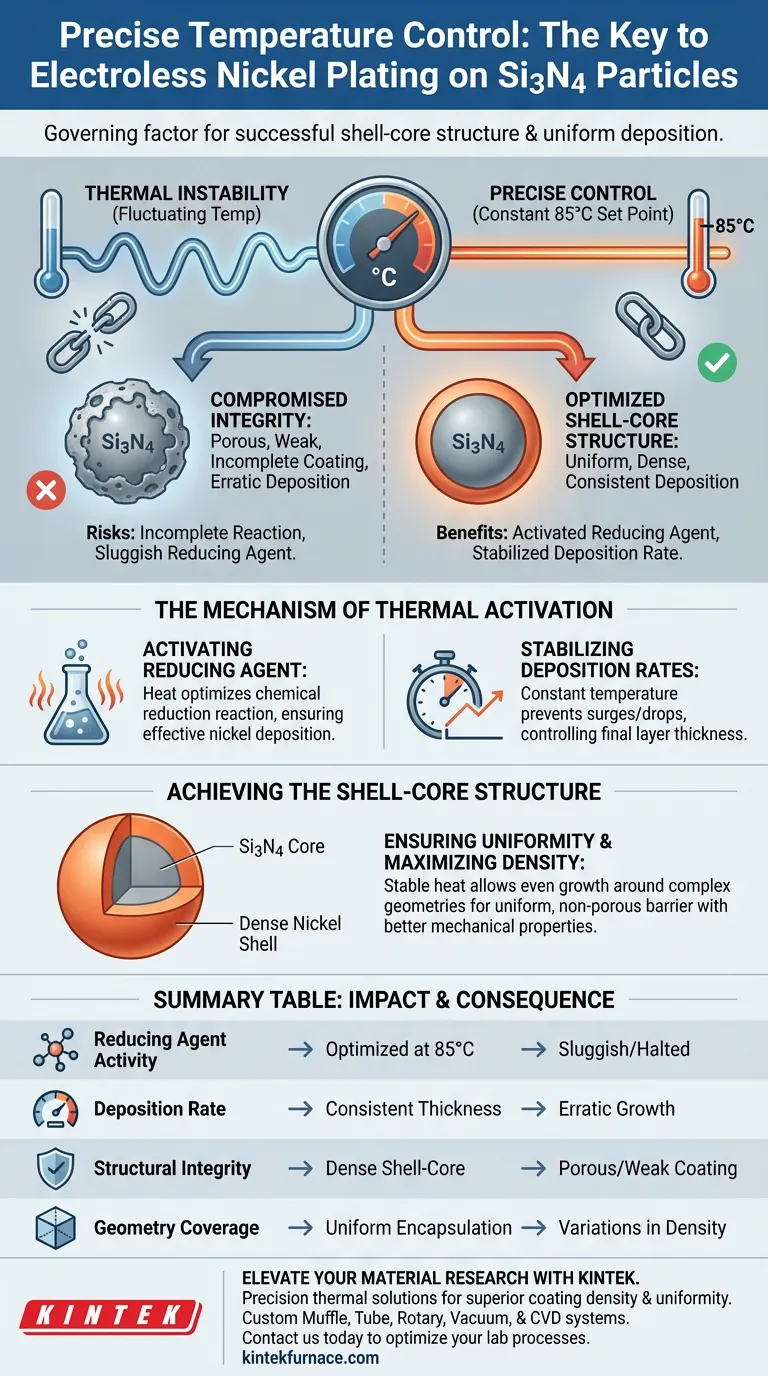
Precise temperature control is the governing factor in the successful electroless nickel plating of silicon nitride (Si3N4) particles. By utilizing heating devices to maintain a specific, constant set point—typically 85°C—you stabilize the chemical reduction reaction. This thermal stability ensures the continuous activity of the reducing agent, resulting in a consistent deposition rate and the formation of a dense, uniform nickel shell around the ceramic core.
Thermodynamic consistency drives structural integrity. In electroless plating, precise thermal management is not just about heating the bath; it is about locking in the reaction rate to guarantee a uniform shell-core structure.

The Mechanism of Thermal Activation
Activating the Reducing Agent
Electroless nickel plating relies on a chemical reduction reaction rather than electrical current. This reaction is highly sensitive to thermal energy.
By heating the solution to a precise level (e.g., 85°C), you ensure the activity of the reducing agent is optimized. Without this specific thermal input, the chemical reaction may be too sluggish to deposit nickel effectively.
Stabilizing Deposition Rates
The speed at which nickel deposits onto the Si3N4 particles is directly proportional to temperature.
Heating devices that maintain a constant temperature ensure a consistent deposition rate. This prevents surges or drops in reaction speed, which are critical for controlling the final thickness of the nickel layer.
Achieving the Shell-Core Structure
Ensuring Uniformity
The ultimate goal of this process is to create a composite material with a "shell-core" structure: the Si3N4 particle is the core, and the nickel is the shell.
Stable temperature control allows the nickel to grow evenly around the complex geometry of the particles. This results in a uniform coating that fully encapsulates the silicon nitride substrate.
Maximizing Coating Density
A fluctuating environment often leads to porous or weak coatings.
By maintaining thermal precision, the reaction fosters the growth of a dense nickel coating. A dense shell provides better mechanical and physical properties for the final composite material.
The Risks of Thermal Instability
Compromised Coating Integrity
If the temperature is allowed to drift, the deposition rate becomes erratic.
This instability can lead to variations in coating thickness or density. The resulting shell may be uneven, failing to provide the intended protection or surface properties to the Si3N4 core.
Incomplete Reaction
Falling below the optimal temperature threshold reduces the energy available for the reaction.
This can cause the reducing agent to lose activity, potentially leading to incomplete coverage of the particles or a halt in the plating process altogether.
Making the Right Choice for Your Goal
To maximize the quality of your plated particles, consider how your thermal management strategy aligns with your specific objectives:
- If your primary focus is coating density: Maintain the temperature strictly at the optimal set point (e.g., 85°C) to ensure the nickel structure creates a solid, non-porous barrier.
- If your primary focus is dimensional uniformity: Use heating devices with high thermal stability to ensure the deposition rate remains constant throughout the entire plating cycle.
Mastering the temperature variable is the single most effective way to transform raw silicon nitride particles into high-performance composite materials.
Summary Table:
| Parameter | Impact of Precise Control | Consequence of Instability |
|---|---|---|
| Reducing Agent Activity | Optimized chemical reaction at 85°C | Sluggish or halted plating process |
| Deposition Rate | Consistent layer thickness and speed | Erratic growth and uneven surfaces |
| Structural Integrity | Dense, non-porous shell-core structure | Porous, weak, or incomplete coating |
| Geometry Coverage | Uniform encapsulation of complex cores | Variations in coating density |
Elevate Your Material Research with KINTEK
Precision is the backbone of successful electroless plating. Backed by expert R&D and manufacturing, KINTEK offers a wide range of high-performance thermal solutions, including Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you are plating silicon nitride particles or developing advanced composite materials, our lab high-temp furnaces are fully customizable to meet your unique temperature stability requirements.
Ready to achieve superior coating density and uniformity? Contact us today to discover how our precision heating equipment can optimize your laboratory processes.
Visual Guide
References
- Yanan Peng, Xiaolei Wang. Water Lubrication of Al-Cu Composites Reinforced by Nickel-Coated Si3N4 Particles. DOI: 10.3390/coatings14020225
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What is the role of a precision annealing furnace in the preparation of ZnO or CuO doped phosphate glass?
- What are the advantages of using a vacuum drying oven for purifying zinc oxide nanoparticles? Superior Material Quality
- What is the primary function of a laboratory electric drying oven in ACBP production? Ensure Precise Pre-treatment
- What is the purpose of adding aluminum in the vacuum distillation process for magnesium? Enhancing Process Stability and Purity
- How does the control of gas flow and reaction time affect NiMo catalyst carbon layers? Master Nanostructure Engineering
- Why is Water Quenching Critical for Metastable Phases in Titanium? Unlock High-Performance Alloy Strength
- What are the technical advantages of using an ALD system over PVD? Achieve Precise Ge:ZnO Thin Film Fabrication
- Why introduce high-purity N2 during cooling after roasting? Preserve Sample Integrity and Prevent Oxidation



















