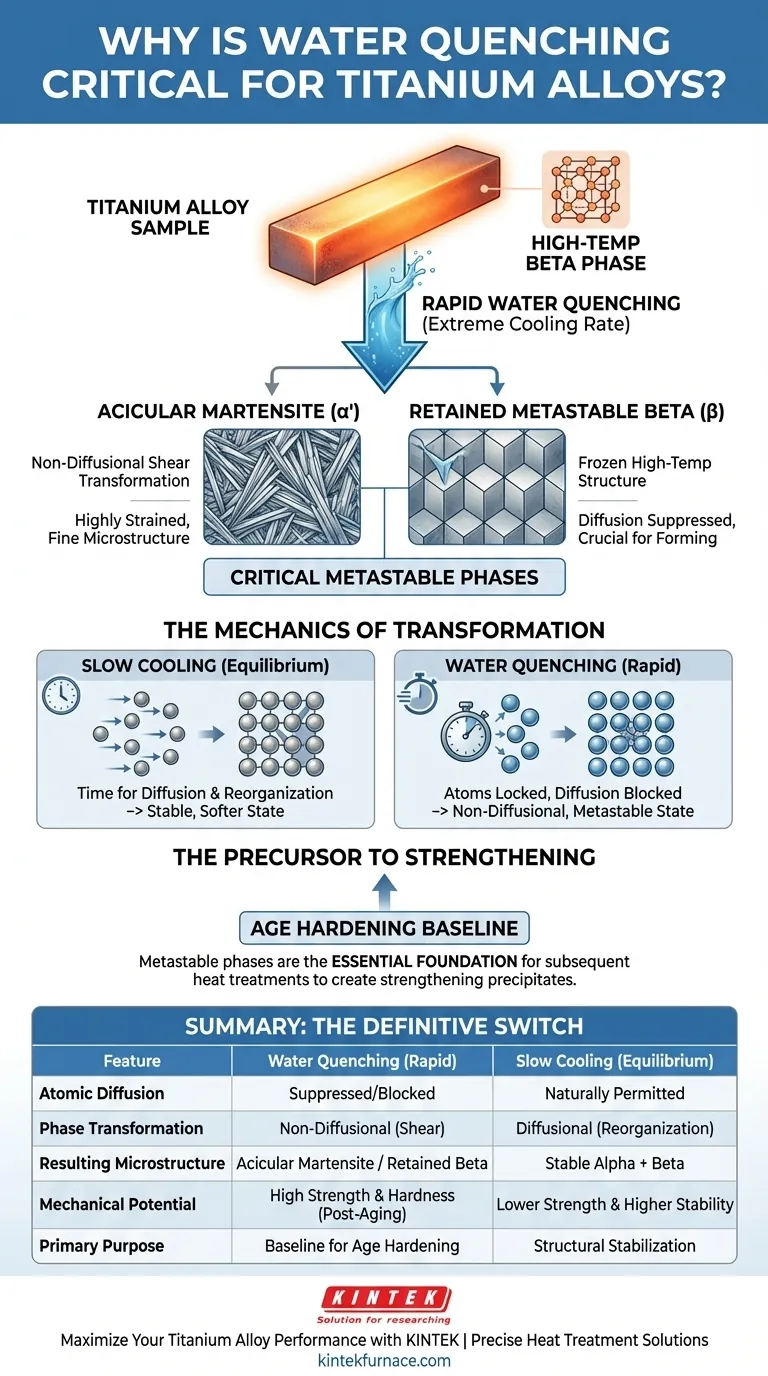
Water Quenching is the definitive thermal mechanism required to lock titanium alloys into high-performance, non-equilibrium states. By delivering an extremely high cooling rate, it physically prevents the alloy’s high-temperature beta-phase structure from naturally transforming into a softer, stable equilibrium state. This thermal shock forces a non-diffusional transformation, which is the only pathway to create the specific microstructures necessary for advanced strengthening.
By suppressing atomic diffusion through rapid cooling, water quenching creates a critical "metastable" condition. It generates acicular martensite or retained beta phases, providing the essential structural foundation required for subsequent age hardening.

The Mechanics of Phase Transformation
Suppressing Diffusion
Titanium alloys naturally seek a stable, equilibrium state as they cool. This natural process requires time for atoms to diffuse and rearrange themselves.
Water quenching interrupts this process by dropping the temperature drastically and immediately. This speed denies the atoms the time necessary to move, effectively locking the high-temperature structure or forcing it to collapse into a new form without diffusion.
Forcing Non-Diffusional Changes
Because diffusion is blocked, the alloy undergoes a non-diffusional phase transformation.
Instead of a slow reorganization, the crystal lattice shears or shifts instantaneously. This is the primary method for generating specific, high-strength phases that cannot exist under slow-cooling conditions.
The Critical Microstructures Created
Forming Acicular Martensite
The most significant result of water quenching in many titanium alloys is the formation of alpha prime ($\alpha'$).
This is an acicular (needle-like) martensite phase. It creates a highly strained, fine microstructure that contributes significantly to the material's potential hardness.
Retaining Metastable Beta
In certain alloy compositions, the quench is fast enough to "freeze" the high-temperature beta phase completely.
This results in a retained metastable beta phase at room temperature. Preserving this phase is often crucial for alloys that require specific forming capabilities or distinct aging responses later in manufacturing.
Understanding the Process Trade-offs
The Necessity of Metastability
The term "metastable" implies a state that is technically unstable but effectively frozen in time.
While an equilibrium state (achieved via slow cooling) is more naturally stable, it generally lacks the mechanical properties required for high-performance engineering. You accept the "instability" of the metastable phase because it is the only route to superior strength.
The Precursor to Strengthening
It is vital to understand that the quenched structure is rarely the final step.
The martensite or retained beta phases serve as the necessary baseline for age hardening. Without the initial water quench to create these specific phases, subsequent heat treatments would fail to produce the desired strengthening precipitates.
Making the Right Choice for Your Goal
To optimize the mechanical properties of titanium alloys, you must align your cooling strategy with your strengthening requirements.
- If your primary focus is Maximum Strength: You must utilize water quenching to convert the beta phase into acicular martensite, setting the stage for effective age hardening.
- If your primary focus is Age Hardening: You must prioritize a cooling rate fast enough to prevent equilibrium, ensuring the retention of metastable phases that respond to aging.
Ultimately, water quenching is not just a cooling method; it is the fundamental switch that activates the alloy's potential for high-strength applications.
Summary Table:
| Feature | Water Quenching (Rapid) | Slow Cooling (Equilibrium) |
|---|---|---|
| Atomic Diffusion | Suppressed/Blocked | Naturally Permitted |
| Phase Transformation | Non-Diffusional (Shear) | Diffusional (Reorganization) |
| Resulting Microstructure | Acicular Martensite ($\alpha'$) / Retained Beta | Stable Alpha + Beta Phases |
| Mechanical Potential | High Strength & Hardness (Post-Aging) | Lower Strength & Higher Stability |
| Primary Purpose | Baseline for Age Hardening | Structural Stabilization |
Maximize Your Titanium Alloy Performance with KINTEK
Precision heat treatment is the foundation of material excellence. At KINTEK, we understand that achieving the perfect metastable phase requires absolute thermal control. Backed by expert R&D and world-class manufacturing, we provide a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems—all customizable to meet your specific lab and high-temperature furnace requirements.
Ready to elevate your metallurgical research and production? Contact us today to discover how our advanced furnace solutions can provide the precise cooling rates and temperature stability your high-strength applications demand.
Visual Guide
References
- Ahmed H. Awad, Shimaa El‐Hadad. Studying the Behavior of Cast and Thermally Treated α + β -Titanium Alloys Using the Abbott Firestone Technique. DOI: 10.1007/s40962-024-01528-w
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering and Brazing Furnace
People Also Ask
- What are the advantages of using microwave drying equipment for organic gels? Preserve Pore Structures Effectively
- What are the limitations of PVD coating? Overcome Challenges for Optimal Surface Engineering
- Why is graphene oxide (GO) essential in microwave synthesis? Unlock Rapid Growth and Precise 2D Nanocomposite Control
- How does a heated substrate platform mitigate the coffee ring effect? Enhance Ag2Se Printing Precision
- Why are specific heating pulses applied when monitoring molten metal surface oscillations? Unlock Material Insights
- What is the purpose of heating the nickel nitrate and biomass mixture to 150°C? Achieve Superior Catalyst Dispersion
- What are the advantages of a crucible furnace? Achieve flexible, low-cost metal melting for small batches
- How does a batch furnace operate and what are its advantages? Boost Precision and Flexibility in Heat Treatment

















