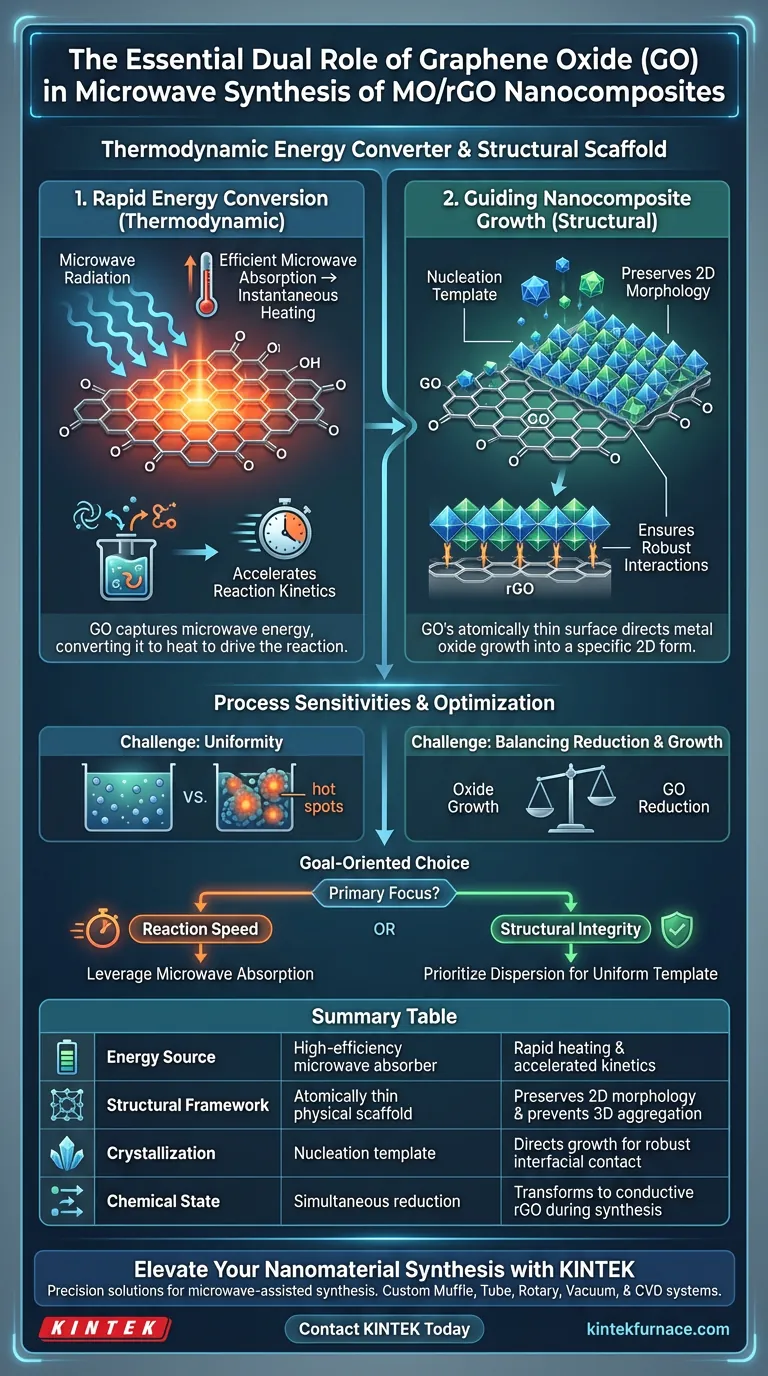
Graphene oxide (GO) serves a distinct, dual-purpose function in the microwave synthesis of metal oxide/reduced graphene oxide (MO/rGO) nanocomposites. It acts first as a high-efficiency microwave absorber, rapidly converting electromagnetic radiation into the thermal energy required to drive the reaction. Simultaneously, it functions as a physical scaffold, using its atomically thin surface to direct the nucleation and growth of metal oxides into a specific two-dimensional morphology.
By combining rapid energy conversion with precise structural templating, GO enables the creation of nanocomposites that are both chemically robust and structurally optimized for high performance.

The Thermodynamic Role: Rapid Energy Conversion
Efficient Microwave Absorption
GO possesses excellent microwave absorption properties. Unlike materials that are transparent to microwaves, GO interacts strongly with the electromagnetic field. This interaction allows it to capture energy efficiently during the synthesis process.
Accelerating Reaction Kinetics
The absorbed electromagnetic energy is rapidly converted into thermal energy. This instantaneous heating accelerates the chemical reaction rates significantly. This speed distinguishes microwave synthesis from slower, conventional heating methods.
The Structural Role: Guiding Nanocomposite Growth
Acting as a Nucleation Template
The atomically thin surface of GO acts as a substrate for the metal oxides. It provides specific sites where the metal oxide crystals can begin to form, or nucleate. This ensures that the metal oxides grow in direct contact with the carbon structure.
Preserving 2D Morphology
Because the metal oxides grow along the GO surface, the final composite retains a two-dimensional shape. The GO essentially molds the metal oxide, preventing it from forming unrestricted, bulk 3D structures.
Ensuring Robust Interactions
The templating process fosters strong connections between layers. By guiding the growth directly on the surface, GO ensures robust interlayer interactions between the metal oxide and the resulting reduced graphene oxide (rGO).
Understanding the Process Sensitivities
The Need for Uniformity
While rapid heating is a benefit, it introduces a challenge regarding control. Because the conversion of energy to heat is so fast, the distribution of GO in the precursor mixture must be perfectly homogeneous. Clumping of GO could lead to "hot spots" and uneven synthesis.
Balancing Reduction and Growth
The process involves simultaneous oxide growth and the reduction of GO to rGO. Achieving the perfect balance requires precise timing. If the reaction is too aggressive, the structural integrity of the resulting rGO template could be compromised.
Making the Right Choice for Your Goal
To maximize the benefits of using GO in microwave synthesis, consider your specific end-goals:
- If your primary focus is reaction speed: Leverage the microwave absorption properties of GO to drastically reduce synthesis time compared to conventional hydrothermal methods.
- If your primary focus is structural integrity: Prioritize the dispersion of GO to ensure the metal oxides have uniform access to the 2D template, securing a consistent morphology.
The utility of graphene oxide lies in its ability to simultaneously fuel the reaction and blueprint the final material's architecture.
Summary Table:
| Feature | Role of Graphene Oxide (GO) | Impact on Synthesis |
|---|---|---|
| Energy Source | High-efficiency microwave absorber | Rapid heating and accelerated reaction kinetics |
| Structural Framework | Atomically thin physical scaffold | Preserves 2D morphology and prevents 3D aggregation |
| Crystallization | Nucleation template | Directs metal oxide growth for robust interfacial contact |
| Chemical State | Undergoes simultaneous reduction | Transforms GO to conductive rGO during synthesis |
Elevate Your Nanomaterial Synthesis with KINTEK
Precision in 2D nanocomposite fabrication requires both advanced material science and high-performance equipment. KINTEK provides the cutting-edge laboratory solutions needed to master the complexities of microwave-assisted synthesis. Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to your specific research requirements.
Whether you are optimizing MO/rGO production or developing next-generation catalysts, our high-temperature furnaces ensure the thermal stability and uniformity your projects demand. Contact KINTEK today to discuss your unique needs and see how our expertise can accelerate your path from the lab to the market.
Visual Guide
References
- Muxuan Yang, Weinan Xu. Scalable solid-state synthesis of 2D transition metal oxide/graphene hybrid materials and their utilization for microsupercapacitors. DOI: 10.1039/d4nr00587b
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
People Also Ask
- Why is high-purity nitrogen (N2) used in MSW pyrolysis? Secure Anaerobic Environments for Maximum Fuel Yield
- What is the role of electric furnaces in the direct reduction of iron? Powering the Future of Green Metallurgy
- How can high-temperature furnace systems be used to evaluate and prevent slagging? Optimize Boiler Performance
- What is the function of the nitrogen source in biomass pyrolysis? Optimize Bio-Oil Yield and Ensure Process Safety
- How does substrate preheating equipment affect the formation and distribution of the Laves phase in Inconel 718?
- Why is the water quenching process necessary for high-entropy alloys? Master Phase Purity and Microstructural Integrity
- What are the specific equipment operational requirements for the SRS process? Unlock Precise Strain Engineering
- What is the primary purpose of high-temperature pyrolysis? Unlock Superior PFAS Removal with Enhanced Hydrophobicity
