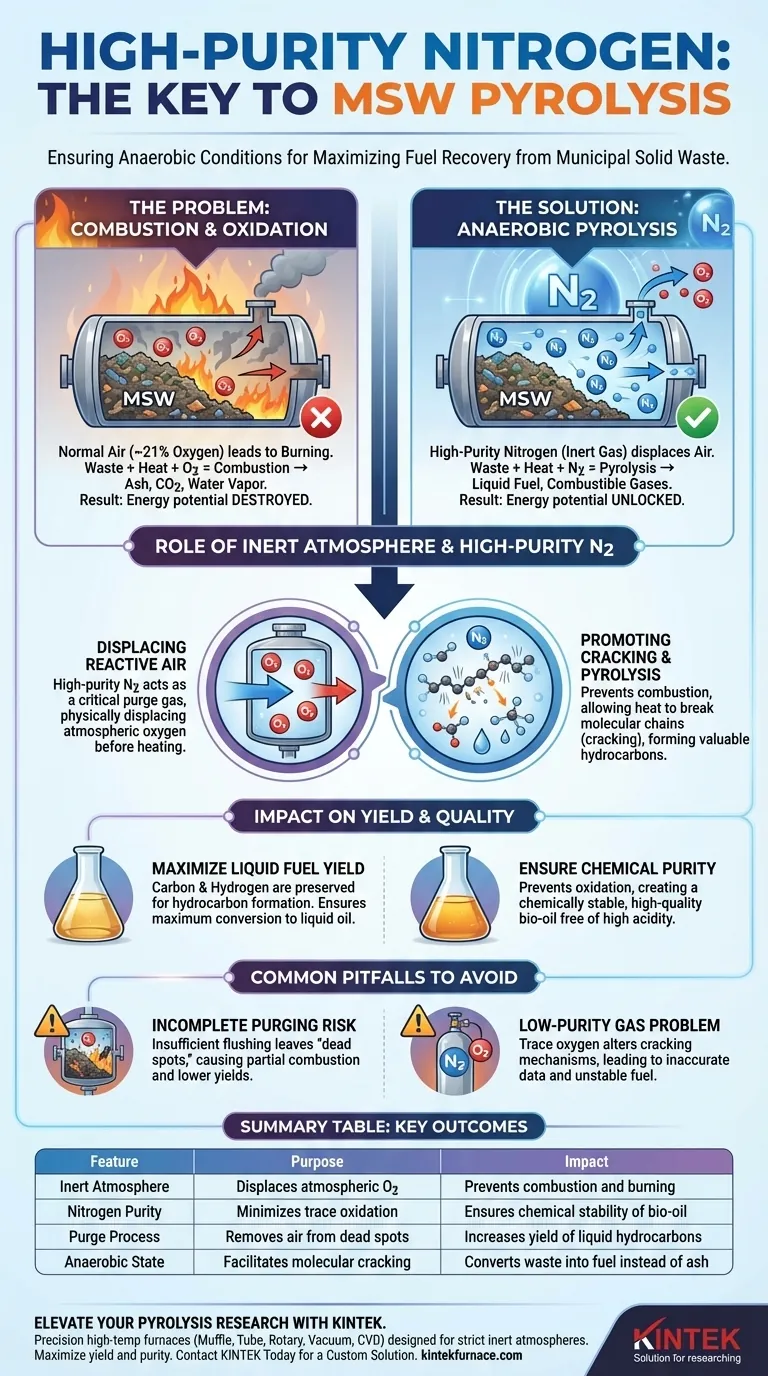
High-purity nitrogen acts as a critical purge gas to establish a strictly anaerobic environment. Before any heating occurs, nitrogen is introduced to physically displace the air within the reactor. This ensures that when temperatures rise, there is no oxygen available to react with the Municipal Solid Waste (MSW).
The fundamental goal of using nitrogen is to force the system into a state of pyrolysis (thermal decomposition) rather than combustion (burning). Without this inert atmosphere, the waste would simply burn, destroying the potential for fuel recovery and producing unwanted ash and carbon dioxide.

The Role of the Inert Atmosphere
Displacing Reactive Air
Normal atmospheric air contains approximately 21% oxygen. If this oxygen remains in the reactor during the heating phase, it will chemically react with the organic material in the MSW.
Establishing Anaerobic Conditions
Nitrogen is used because it is an inert gas, meaning it does not chemically react with the waste material under these conditions. By flooding the system with high-purity nitrogen, you effectively create a "blanket" that insulates the waste from oxidation.
Controlling the Chemical Reaction
Preventing Combustion
The presence of oxygen at high temperatures triggers combustion. This process releases energy but consumes the carbon and hydrogen atoms needed to create fuel, converting them instead into CO2 and water vapor.
Promoting Cracking and Pyrolysis
When oxygen is removed, the heat energy cannot cause burning. Instead, the energy causes the long molecular chains within the waste to vibrate and break apart.
This process, known as cracking, creates smaller, valuable molecules. These molecules condense into liquid fuel and combustible gases, which is the primary objective of pyrolysis.
Impact on Yield and Quality
Maximizing Liquid Fuel Yield
Because the carbon and hydrogen are not being consumed by fire, they remain available to form hydrocarbons. High-purity nitrogen ensures the maximum possible conversion of solid waste into liquid oil.
Ensuring Chemical Purity
If oxidation occurs, the chemical composition of the resulting bio-oil changes, often resulting in high acidity or instability. An oxygen-free environment maintains the chemical quality of the fuel.
Common Pitfalls to Avoid
The Risk of Incomplete Purging
Simply introducing nitrogen is not enough; the volume must be sufficient to thoroughly flush out "dead spots" in the reactor. Failure to displace all air results in partial combustion, which manifests as charred material and lower liquid yields.
The Problem with Low-Purity Gas
Using nitrogen with trace amounts of oxygen can compromise experimental data. In scientific experiments, even small amounts of oxidation can alter the specific cracking mechanisms, leading to inaccurate conclusions about the MSW's energy potential.
Making the Right Choice for Your Goal
To maximize the effectiveness of your pyrolysis experiment, consider the following approach regarding nitrogen flow:
- If your primary focus is Fuel Quantity: Ensure a high flow rate during the pre-heat purge to guarantee 0% oxygen presence, maximizing the carbon available for oil production.
- If your primary focus is Chemical Stability: Use the highest purity grade of nitrogen available to prevent micro-oxidation that could lower the caloric value of your liquid fuel.
The integrity of your inert atmosphere is the single greatest determinant of whether you produce valuable fuel or simply burn waste.
Summary Table:
| Feature | Purpose in MSW Pyrolysis | Impact on Outcome |
|---|---|---|
| Inert Atmosphere | Displaces atmospheric oxygen | Prevents combustion and burning |
| Nitrogen Purity | Minimizes trace oxidation | Ensures chemical stability of bio-oil |
| Purge Process | Removes air from dead spots | Increases yield of liquid hydrocarbons |
| Anaerobic State | Facilitates molecular cracking | Converts waste into fuel instead of ash |
Elevate Your Pyrolysis Research with KINTEK
Precision in pyrolysis starts with a controlled environment. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to maintain the strict inert atmospheres required for Municipal Solid Waste (MSW) research.
Whether you need to maximize liquid fuel yield or ensure the chemical purity of your bio-oil, our lab high-temp furnaces are fully customizable to meet your unique experimental needs. Don't let oxidation compromise your data—partner with the leaders in thermal processing.
Contact KINTEK Today for a Custom Solution
Visual Guide
References
- Indra Mamad Gandidi, Arinal Hamni. Integrated two-step co-pyrolysis under several low-cost natural catalysts to produce aromatic-rich liquid fuel from mixed municipal solid waste. DOI: 10.1093/ce/zkae092
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How do surface oxidation systems improve the interface performance of graphitized fibers? Maximize Composite Strength
- How does controlled thermal treatment affect delta-MnO2? Optimize Porosity & Surface Area for Better Battery Performance
- Why is a slow heating rate utilized for rice husk biochar? Optimize Pore Structure and Adsorption Performance
- What is the primary role of a ball mill in raw material preparation for vacuum carbothermic reduction of magnesium? Ensure a Complete and Rapid Reaction
- What role does a closed pressure vessel play during the carbonation of gamma-C2S? Unlock Rapid Mineralization
- What are the primary advantages of using a downdraft fixed-bed reactor for co-gasification? Pure Syngas Made Simple
- How is a mass spectrometer utilized in TPO tests for catalysts? Enhance Your Material Characterization
- What are the primary purposes of using high-purity argon flow during the pyrolysis of CMS membranes? Achieve High-Purity Results



















