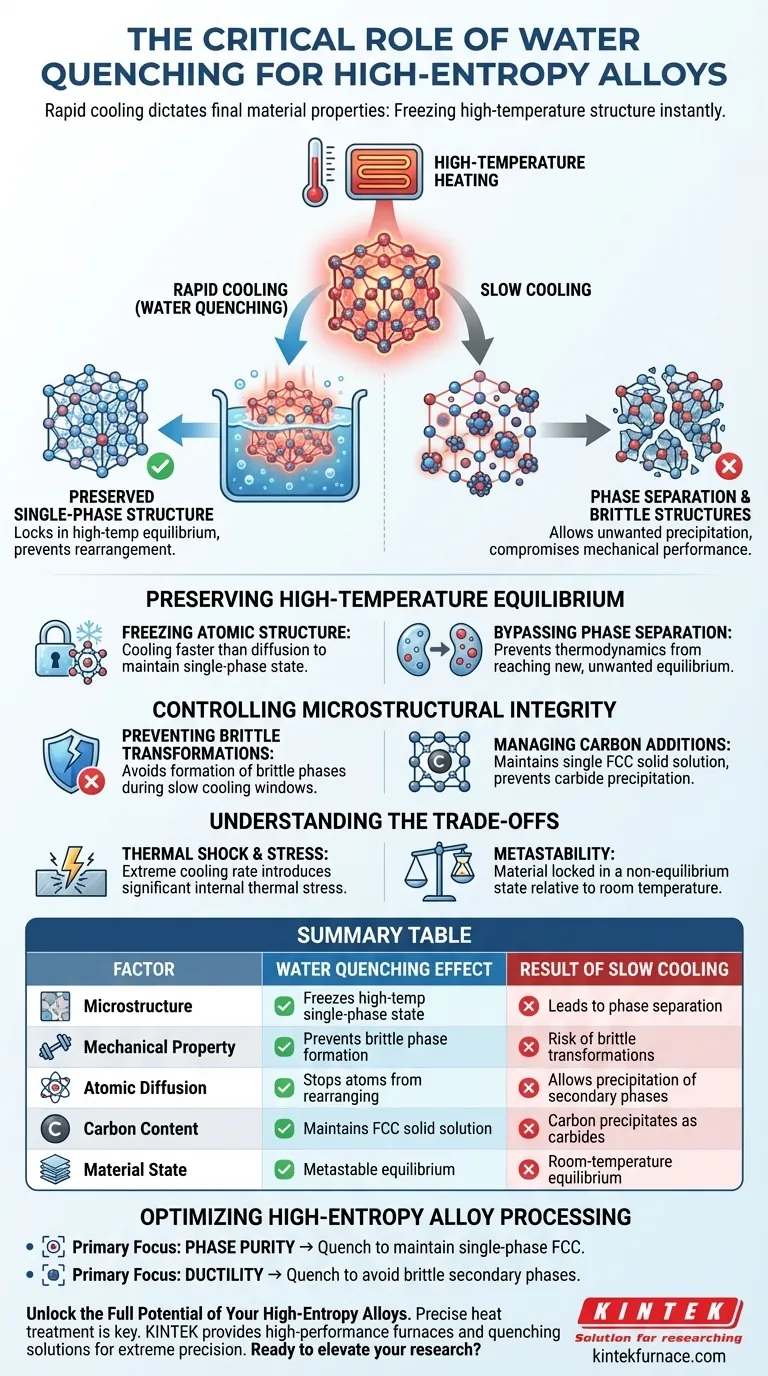
Rapid cooling dictates the final material properties. Water quenching is a critical step for high-entropy alloys (HEAs) because it utilizes an extremely fast cooling rate to "freeze" the high-temperature structure instantly. This prevents the alloy from naturally separating into unwanted secondary phases or brittle structures, which typically occurs during a slower cooling process.
The water quenching process effectively locks the alloy in a high-temperature equilibrium state at room temperature. By denying the atoms time to rearrange, it ensures the preservation of a desired single-phase structure and prevents the precipitation of detrimental phases that compromise mechanical performance.
Preserving High-Temperature Equilibrium
The primary goal of heating an HEA is often to achieve a uniform, single-phase structure. Quenching is the mechanism used to retain that state.
Freezing the Atomic Structure
At high temperatures, HEAs often exist in a single-phase or equilibrium state.
To maintain this state at room temperature, the cooling process must be faster than the rate at which atoms can diffuse and rearrange. Water quenching provides this speed, effectively locking the high-temperature atomic configuration in place.
Bypassing Phase Separation
If an alloy is allowed to cool slowly, the thermodynamics of the material change.
Slow cooling gives the material time to reach a new equilibrium, which often involves the separation of elements. This leads to the precipitation of unwanted secondary phases that ruin the homogeneity of the alloy.
Controlling Microstructural Integrity
Beyond simply freezing the structure, quenching allows for precise engineering of the alloy's mechanical characteristics by managing specific chemical interactions.
Preventing Brittle Transformations
Slow cooling windows are often where brittle phase transformations occur.
By rapidly bypassing this temperature window, the alloy avoids forming these brittle structures. This is essential for ensuring the final material retains toughness rather than becoming prone to fracture.
Managing Carbon Additions
The reference specifically notes the challenge of alloys containing carbon.
Without quenching, carbon tends to precipitate out of the solution. Water quenching ensures the maintenance of a single FCC (Face-Centered Cubic) solid solution structure, keeping the carbon integrated within the lattice rather than forming separate carbides.
Understanding the Trade-offs
While water quenching is necessary for specific microstructures, it introduces physical challenges that must be managed.
Thermal Shock and Stress
The defining feature of this process is the extremely fast cooling rate.
While this protects the microstructure, the rapid temperature drop introduces significant thermal stress. This can lead to internal residual stresses within the material if not accounted for in subsequent processing steps.
Metastability
Quenching creates a state that is stable at room temperature but is technically metastable.
You are forcing the material to exist in a state it usually only holds at high temperatures. While this prevents unwanted precipitates, it means the material is locked in a non-equilibrium state relative to room temperature thermodynamics.
Optimizing High-Entropy Alloy Processing
The decision to water quench is ultimately a decision about the phase purity of your final material.
- If your primary focus is Phase Purity: Quenching is mandatory to maintain a single-phase FCC structure, specifically preventing element separation.
- If your primary focus is Ductility: You must quench to avoid the formation of brittle secondary phases that develop during slow cooling.
Water quenching transforms a theoretical high-temperature structure into a practical, room-temperature reality.
Summary Table:
| Factor | Water Quenching Effect | Result of Slow Cooling |
|---|---|---|
| Microstructure | Freezes high-temp single-phase state | Leads to phase separation |
| Mechanical Property | Prevents brittle phase formation | Risk of brittle transformations |
| Atomic Diffusion | Stops atoms from rearranging | Allows precipitation of secondary phases |
| Carbon Content | Maintains FCC solid solution | Carbon precipitates as carbides |
| Material State | Metastable equilibrium | Room-temperature equilibrium |
Unlock the Full Potential of Your High-Entropy Alloys
Precise heat treatment is the difference between a breakthrough material and a brittle failure. At KINTEK, we understand the rigorous demands of HEA processing. Backed by expert R&D and world-class manufacturing, we provide high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems tailored for extreme precision. Whether you need customizable high-temp furnaces or rapid-response quenching solutions, our equipment ensures your materials achieve the perfect single-phase structure every time.
Ready to elevate your material science research? Contact us today to find your custom furnace solution!
Visual Guide
References
- Yukun Lv, Jian Chen. Improving Mechanical Properties of Co-Cr-Fe-Ni High Entropy Alloy via C and Mo Microalloying. DOI: 10.3390/ma17020529
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What role does an electric thermostatic drying oven play in the pre-treatment of Fe–Ni/AC catalysts? Essential Guide
- Why is a vacuum oven preferred for drying MXene-modified electrodes? Optimize Your Lab's Electrochemical Success
- How does a high-precision infrared temperature measurement system influence the sintering quality of Al2O3/TiC ceramics?
- Why Use a Vacuum Oven for Cu-Cu2O/g-C3N4 Catalysts? Preserve Purity and Structural Integrity
- What advantages does AlMe2iPrO (DMAI) offer over Trimethylaluminum (TMA)? Achieve Superior Area Selectivity
- What additional benefits do vacuum chambers provide beyond environmental control? Enhance Material Purity and Process Efficiency
- What is the primary role of a carbonization curing chamber? Unlock High Strength in Magnesium Slag Mortar
- What is the significance of using a hydrogen etching process in a reaction chamber? Mastering SiC Surface Preparation



















