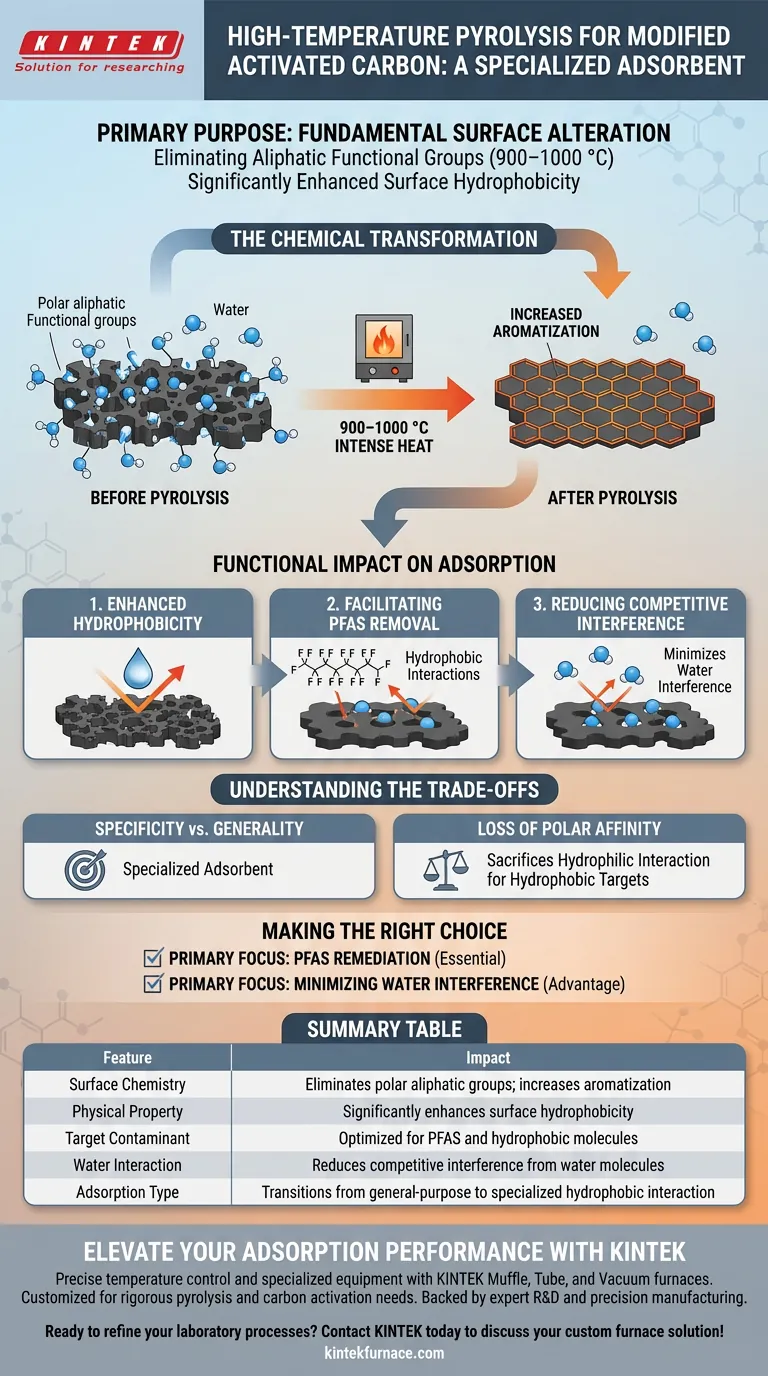
The primary purpose of high-temperature pyrolysis is to fundamentally alter the surface chemistry of activated carbon by eliminating aliphatic functional groups. Conducted at temperatures between 900–1000 °C, this process drives a transition toward a more aromatic structure, directly resulting in significantly enhanced surface hydrophobicity.
By stripping away polar, water-attracting groups and increasing aromatization, high-temperature pyrolysis creates a specialized adsorbent optimized for hydrophobic interactions, specifically targeting contaminants like PFAS while minimizing interference from water.
The Chemical Transformation
Elimination of Functional Groups
The intense heat serves as a precise mechanism for surface modification. Its main function is to strip away aliphatic functional groups that naturally reside on the carbon surface.
Increasing Aromatization
As these aliphatic groups are removed, the carbon lattice undergoes a structural reorganization. This process increases the degree of aromatization, resulting in a more ordered and stable carbon framework.
Functional Impact on Adsorption
Enhancing Hydrophobicity
The chemical changes induced by pyrolysis lead to a distinct physical property: increased hydrophobicity. The treated surface becomes highly effective at repelling water, which is a critical trait for specific adsorption tasks.
Facilitating PFAS Removal
This hydrophobic nature makes the modified carbon particularly effective at capturing PFAS molecules. The adsorption occurs primarily through hydrophobic interactions, allowing the carbon to latch onto these persistent contaminants more efficiently.
Reducing Competitive Interference
Standard activated carbon often contains polar functional groups that attract water molecules. By removing these groups, pyrolysis significantly reduces the competition from water molecules, ensuring that the adsorption sites remain available for the target contaminants.
Understanding the Trade-offs
Specificity vs. Generality
It is important to recognize that this process is a form of specialization. By maximizing hydrophobicity to target substances like PFAS, you are intentionally altering the carbon's broad-spectrum properties.
Loss of Polar Affinity
The removal of polar functional groups is beneficial for hydrophobic targets but reduces the material's affinity for polar substances. This modification sacrifices the ability to interact with hydrophilic compounds in exchange for superior performance against hydrophobic threats.
Making the Right Choice for Your Goal
When deciding whether to utilize activated carbon modified by high-temperature pyrolysis, consider your specific target contaminants.
- If your primary focus is PFAS remediation: This process is essential, as it maximizes the hydrophobic interactions required to capture these difficult molecules.
- If your primary focus is minimizing water interference: This method provides a clear advantage by removing the polar groups that typically attract water and block adsorption sites.
High-temperature pyrolysis transforms activated carbon from a general adsorbent into a highly specialized tool for hydrophobic contaminant removal.
Summary Table:
| Feature | Impact of High-Temperature Pyrolysis (900–1000 °C) |
|---|---|
| Surface Chemistry | Eliminates polar aliphatic groups; increases aromatization |
| Physical Property | Significantly enhances surface hydrophobicity |
| Target Contaminant | Optimized for PFAS and hydrophobic molecules |
| Water Interaction | Reduces competitive interference from water molecules |
| Adsorption Type | Transitions from general-purpose to specialized hydrophobic interaction |
Elevate Your Adsorption Performance with KINTEK
Precise surface modification requires exact temperature control and specialized equipment. KINTEK provides industry-leading Muffle, Tube, and Vacuum furnaces designed to meet the rigorous demands of high-temperature pyrolysis and carbon activation.
Backed by expert R&D and precision manufacturing, our systems are fully customizable to your unique material science needs. Whether you are optimizing PFAS remediation or developing advanced adsorbents, KINTEK delivers the thermal stability you need for consistent results.
Ready to refine your laboratory processes? Contact KINTEK today to discuss your custom furnace solution!
Visual Guide
References
- Md Manik Mian, Shubo Deng. Recent advances in activated carbon driven PFAS removal: structure-adsorption relationship and new adsorption mechanisms. DOI: 10.1007/s11783-025-1998-3
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the role of industrial thermometers in monitoring thermal stress? Ensure Safety via High-Precision Data
- What mechanisms generate heat in induction heating? Discover the Science of Efficient Material Processing
- What is the function of the circulation pump in a salt bath furnace? Master Sorbite Transformation Quality
- What type of furnace is used for heat treatment? Choose the Right Solution for Your Materials
- What are the technical functions of carrier gases in VTD? Master Vapor Transport Deposition Control
- How does a laboratory furnace operate? Master Heating Principles for Your Lab
- What are the advantages of using multi-stage laboratory sintering furnaces? Ensure Defect-Free Powder Metallurgy
- What is the function of the slow cooling feature in a furnace for Li2.7Sc0.1Sb? Master Single-Crystal Quality



















