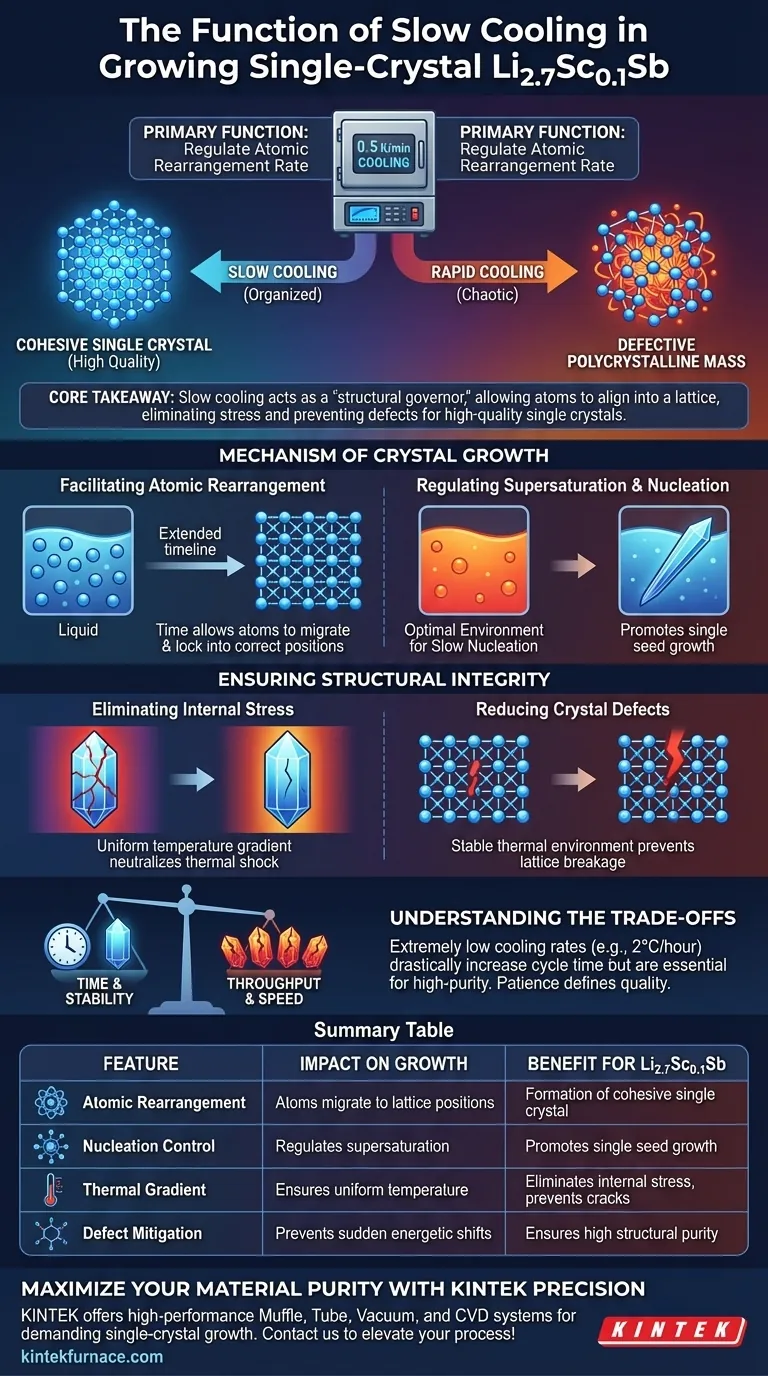
The primary function of the slow cooling feature in a programmable temperature control furnace is to regulate the rate of atomic rearrangement during the crystallization process. For a material like single-crystal Li2.7Sc0.1Sb, this precise control (typically around 0.5 K/min) is the critical factor that determines whether the final product forms a cohesive, high-quality single crystal or a defective, polycrystalline mass.
Core Takeaway The slow cooling process acts as a "structural governor," providing the necessary time for atoms to align perfectly into a lattice structure during phase transitions. This eliminates internal thermal stress and prevents defects, directly enabling the growth of large-sized, high-quality single crystals.

The Mechanism of Crystal Growth
Facilitating Atomic Rearrangement
To grow a single crystal, the raw material must transition from a liquid or disordered phase into a highly ordered solid phase.
Time is the critical variable here. The slow cooling feature extends the duration of this transition.
By cooling at a controlled rate, such as 0.5 K/min, you provide the atoms sufficient time to migrate and lock into their correct positions within the crystal lattice. If cooling occurs too quickly, atoms become "frozen" in place before they can organize, leading to structural chaos.
Regulating Supersaturation and Nucleation
Precise temperature control creates an optimal environment for slow nucleation.
As the temperature drops from a molten state (e.g., cooling down from a high soaking temperature), the solution becomes supersaturated.
Slow cooling ensures this supersaturation happens gradually. This promotes the growth of a single, high-quality crystal seed (such as needle-like structures) rather than triggering the simultaneous, rapid formation of multiple crystals, which results in a polycrystalline or amorphous product.
Ensuring Structural Integrity
Eliminating Internal Stress
Rapid temperature changes induce significant thermal shock within a material.
In single-crystal growth, uneven cooling causes different parts of the crystal to contract at different rates. This generates internal stress, which can lead to cracks or fractures once the crystal creates its final form.
Programmable slow cooling ensures the temperature gradient remains uniform throughout the sample, effectively neutralizing these thermal stresses before they become permanent.
Reducing Crystal Defects
Defects occur when the lattice structure is interrupted or misaligned.
The primary reference indicates that slow cooling is vital for reducing crystal defects. By maintaining a stable thermal environment, the furnace prevents sudden energetic shifts that would otherwise force the crystal lattice to break or deform during growth.
Understanding the Trade-offs
Time vs. Throughput
The most significant trade-off in this process is time.
Achieving the high stability required for large-diameter crystals often necessitates extremely low cooling rates—sometimes as slow as 2°C per hour in similar contexts.
While this drastically increases the total cycle time (potentially extending the process over several days), it is a non-negotiable cost for achieving high-purity single crystals. Rushing this stage to save time will almost invariably sacrifice the structural integrity of the Li2.7Sc0.1Sb sample.
Making the Right Choice for Your Goal
When programming your furnace profile, your cooling rate should be dictated by your specific requirements for the Li2.7Sc0.1Sb crystal.
- If your primary focus is Crystal Size and Purity: Prioritize an extremely slow cooling rate (e.g., 0.5 K/min or slower) to minimize stress and maximize atomic order.
- If your primary focus is Process Speed: You may increase the cooling rate, but you must accept a higher probability of polycrystalline formation and internal defects.
Ultimately, the quality of your single crystal is defined by the patience of your cooling cycle.
Summary Table:
| Feature of Slow Cooling | Impact on Crystal Growth | Benefit for Li2.7Sc0.1Sb |
|---|---|---|
| Atomic Rearrangement | Provides time for atoms to migrate to lattice positions | Formation of a cohesive single crystal vs. polycrystalline mass |
| Nucleation Control | Regulates supersaturation levels | Promotes growth of a single seed rather than multiple sites |
| Thermal Gradient | Ensures uniform temperature distribution | Eliminates internal stress and prevents cracks/fractures |
| Defect Mitigation | Prevents sudden energetic shifts during phase change | Ensures high structural purity and lattice alignment |
Maximize Your Material Purity with KINTEK Precision
Achieving the perfect 0.5 K/min cooling rate for Li2.7Sc0.1Sb requires more than just a furnace; it requires precision engineering. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Vacuum, and CVD systems designed for the most demanding single-crystal growth applications. Whether you need standard lab equipment or a fully customizable high-temperature furnace tailored to your unique research needs, our systems deliver the thermal stability you demand.
Ready to elevate your crystal growth process? Contact KINTEK today to consult with our experts!
Visual Guide
References
- Jingwen Jiang, Thomas F. Fässler. Scandium Induced Structural Disorder and Vacancy Engineering in Li<sub>3</sub>Sb – Superior Ionic Conductivity in Li<sub>3−3</sub><i><sub>x</sub></i>Sc<i><sub>x</sub></i>Sb. DOI: 10.1002/aenm.202500683
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What are advanced materials and composites? Unlock Superior Performance for Your Innovations
- What are the functions of an industrial drying furnace vertically installed below a shredder? Efficient LIB Recycling
- What is the function of the heating device in the micro-Kjeldahl method? Master Protein Analysis in Mushrooms
- Why is thermal insulation applied to cylindrical components in thermal stress tests? Enhance Calculation Precision
- What is the importance of a stable thermal environment during crystallization? Ensure Precision in Metal Oxide Films
- How is vacuum typically defined in practical terms? Understanding Pressure Reduction for Your Applications
- Why is a high-precision mass flow controller essential for long-term restart performance testing of catalysts?
- What are the main advantages of crucible furnaces? Unmatched Flexibility for Small-Scale Melting



















