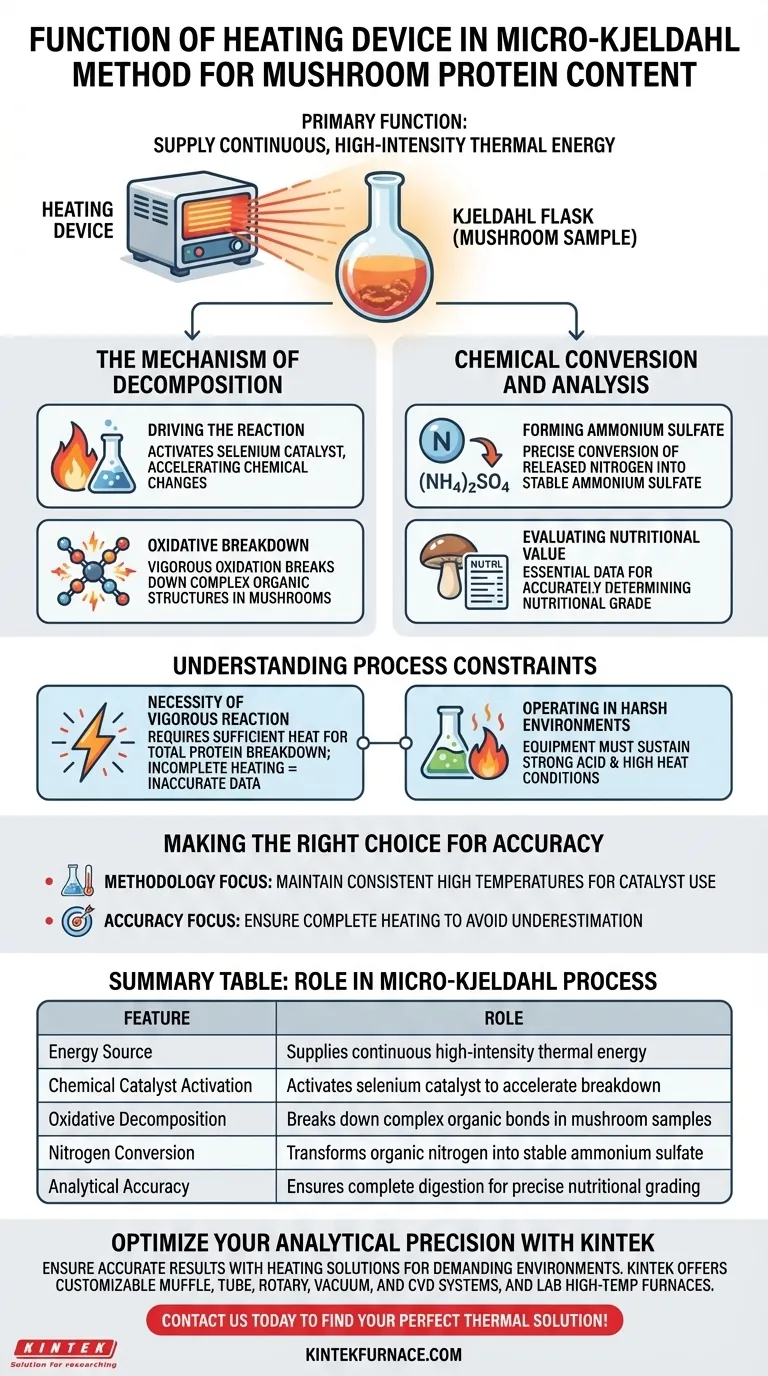
The primary function of the heating device in the micro-Kjeldahl method is to supply continuous, high-intensity thermal energy to the mushroom sample within the flask. This energy is the driving force behind the entire analysis, initiating and sustaining the chemical reactions necessary to break down complex organic structures. Without this sustained thermal input, the reagents would remain inactive, and the protein content could not be measured.
In the micro-Kjeldahl method, the heating device acts as the engine for chemical decomposition. It drives the vigorous oxidation required to convert the organic nitrogen found in mushrooms into ammonium sulfate, enabling precise nutritional quantification.

The Mechanism of Decomposition
Driving the Reaction
The heating device ensures the environment inside the Kjeldahl flask reaches the critical energy levels required for a chemical reaction.
It typically operates in conjunction with a selenium catalyst.
The heat activates this system, accelerating the chemical changes that must occur for the analysis to proceed.
Oxidative Breakdown
The ultimate goal of applying this heat is to achieve vigorous oxidative decomposition.
Mushrooms contain complex proteins and other nitrogenous organic compounds.
The high thermal energy forces the bonds within these compounds to break apart, effectively dismantling the sample's organic structure.
Chemical Conversion and Analysis
Forming Ammonium Sulfate
The specific chemical outcome of this heating process is the precise conversion of nitrogen.
As the organic matter decomposes, the nitrogen it holds is released and transformed into ammonium sulfate.
This conversion is the pivot point of the method, turning complex biological structures into a stable chemical compound that can be measured.
Evaluating Nutritional Value
This heating step is not merely a preparatory phase; it is fundamental to the entire analytical process.
By ensuring total decomposition, the method allows for a quantitative evaluation of the mushroom.
This data is essential for accurately determining the nutritional grade of edible mushrooms.
Understanding the Process Constraints
The Necessity of Vigorous Reaction
The micro-Kjeldahl method relies on vigorous decomposition, not passive separation.
A common pitfall is underestimating the amount of energy required; insufficient heat will fail to fully break down the proteins.
This leads to incomplete conversion of nitrogen, resulting in inaccurate data.
Operating in Harsh Environments
The heating device must function effectively within a strong acid environment.
This combination of high heat and high acidity creates the aggressive conditions necessary for total breakdown.
However, it also imposes a constraint: the process requires equipment capable of sustaining these harsh conditions without failure.
Making the Right Choice for Your Goal
To ensure accurate protein determination in mushrooms, consider the following:
- If your primary focus is Methodology: Ensure the heating device maintains consistent, high temperatures to effectively utilize the selenium catalyst.
- If your primary focus is Accuracy: Recognize that incomplete heating leads to partial decomposition, directly resulting in an underestimation of protein content.
Mastering the heating phase ensures the conversion of nitrogen is complete, providing the bedrock for accurate nutritional assessment.
Summary Table:
| Feature | Role in Micro-Kjeldahl Process |
|---|---|
| Energy Source | Supplies continuous high-intensity thermal energy |
| Chemical Catalyst Activation | Activates selenium catalyst to accelerate breakdown |
| Oxidative Decomposition | Breaks down complex organic bonds in mushroom samples |
| Nitrogen Conversion | Transforms organic nitrogen into stable ammonium sulfate |
| Analytical Accuracy | Ensures complete digestion for precise nutritional grading |
Optimize Your Analytical Precision with KINTEK
Ensure your protein determination yields accurate, repeatable results with heating solutions designed for the most demanding laboratory environments. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized lab high-temp furnaces—all fully customizable to meet your unique chemical decomposition needs.
Don't let incomplete digestion compromise your data. Whether you are performing nutritional grading or complex organic analysis, our robust equipment is built to withstand strong acid environments and deliver the thermal consistency you require.
Ready to elevate your lab's performance? Contact us today to find your perfect thermal solution!
Visual Guide
References
- Arowora Kayode Adebisi, Isaac John Umaru. Comparative Study on the Proximate and Amino Acids Levels in Selected Edible Mushroom Species. DOI: 10.58578/ajbmbr.v2i2.5892
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the purpose of hydrogen pre-treatment for Ni-Co doped carbon nanotubes? Unlock Superior Catalyst Activation
- What are the advantages of using a vacuum drying oven for MnMgPO4@C3N4? Preserving Photocatalyst Integrity
- Why is the water quenching process necessary for high-entropy alloys? Master Phase Purity and Microstructural Integrity
- What long-term considerations are important when selecting a kiln? Ensure Cost-Effective, Compliant Operations
- How does a symmetric suction design improve steel wire heat treatment? Achieve Uniform Salt Flow and Sorbite Quality
- What is the function of an industrial electric furnace in Al-Cu 224 alloy preparation? Optimize Your Metal Production
- What is the purpose of applying a hexagonal Boron Nitride (h-BN) coating to graphite? Enhance Purity & Tool Longevity
- What is the mechanism of the steam and air mixture used in the decoking process? Essential High-Temp Reaction Guide



















