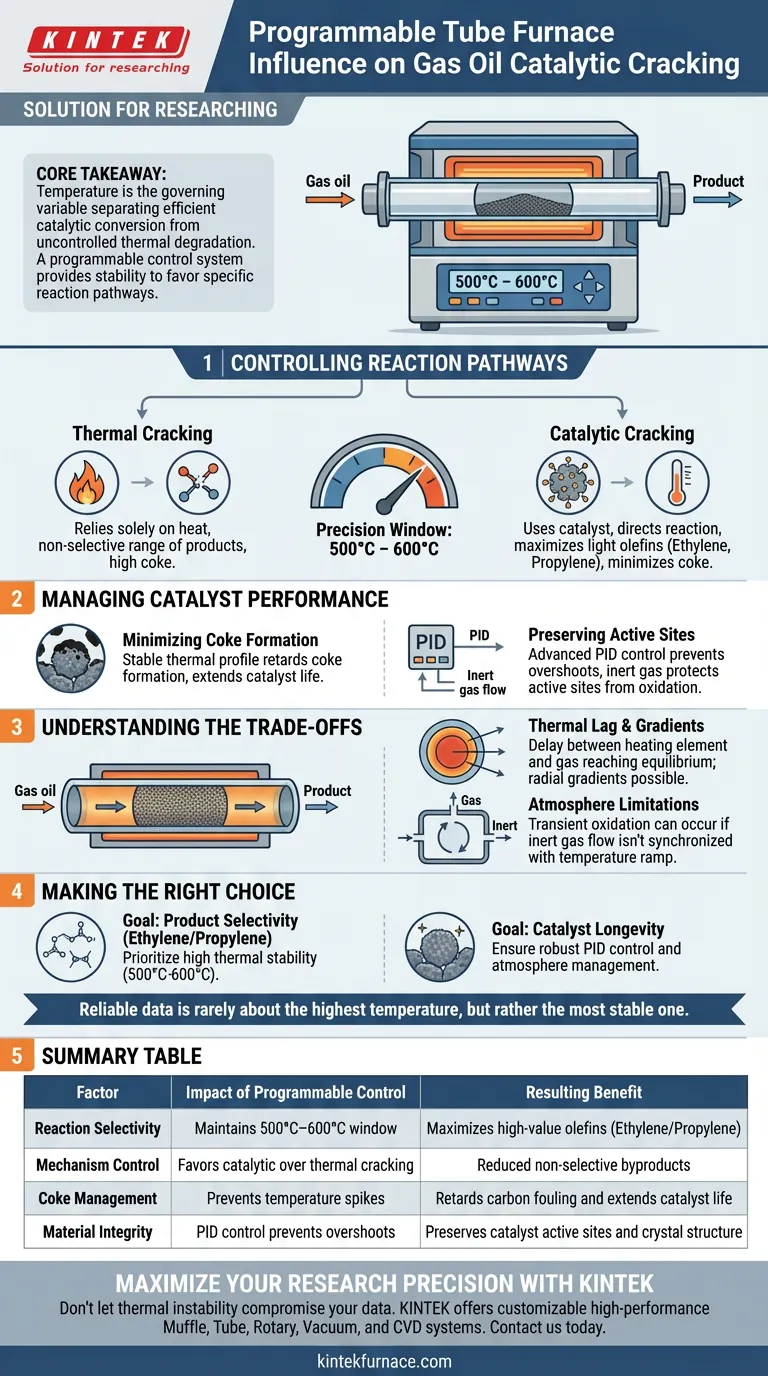
A programmable tube furnace serves as the critical regulator of reaction selectivity and catalyst longevity in gas oil cracking. By strictly maintaining temperatures within the 500°C to 600°C range, the system dictates the ratio of thermal cracking to catalytic cracking, directly influencing the yield of high-value olefins like ethylene and propylene while minimizing coke deposition.
Core Takeaway Temperature is the governing variable that separates efficient catalytic conversion from uncontrolled thermal degradation. A programmable control system provides the stability required to favor specific reaction pathways, ensuring that the catalyst's active sites dictate the product distribution rather than random thermal energy.

Controlling Reaction Pathways
Balancing Thermal and Catalytic Mechanisms
In gas oil cracking, two distinct mechanisms compete: thermal cracking and catalytic cracking.
Thermal cracking relies solely on heat to break chemical bonds, often resulting in a wide, non-selective range of products.
Catalytic cracking uses a catalyst to lower activation energy, directing the reaction toward specific products.
The Role of Precision Temperature
The furnace's programmable control allows you to operate in a precise window, typically 500°C to 600°C.
By locking in the temperature, the system ensures the reaction is driven by the catalyst's properties rather than excessive thermal energy.
This precision is what maximizes the selectivity of desired light olefins, such as ethylene and propylene, preventing the formation of unwanted byproducts.
Managing Catalyst Performance
Minimizing Coke Formation
One of the primary failure modes in catalytic cracking is the accumulation of coke (carbon deposits) on the catalyst surface.
Coke formation is highly sensitive to temperature fluctuations; excessive heat accelerates the rate at which carbon fouls the active sites.
A programmable system maintains a stable thermal profile, effectively retarding the rate of coke formation and extending the useful life of the catalyst within the 10mm reaction tube.
Preserving Active Sites
Beyond the cracking reaction itself, the furnace plays a role in maintaining the catalyst's material integrity.
Advanced systems utilize PID (Proportional-Integral-Derivative) control to prevent temperature overshoots that could oxidize or alter the catalyst's crystal structure.
When combined with controlled atmospheres (using gases like Nitrogen or Argon), the furnace protects the catalyst's surface active sites from degradation during high-temperature phases.
Understanding the Trade-offs
Thermal Lag and Gradients
While the programmable controller may display a precise temperature, the internal environment of the tube can differ.
There is often a delay between the heating element reaching the setpoint and the gas oil vapor reaching equilibrium.
Furthermore, even in a narrow 10mm tube, radial temperature gradients can exist, meaning the gas near the walls may be hotter than the gas in the center, potentially affecting reproducibility.
Atmosphere Limitations
A tube furnace is excellent for small-scale, controlled atmosphere experiments, but it is a closed system.
If the flow of inert gas is not perfectly synchronized with the temperature ramp, transient oxidation can still occur before the reducing environment is fully established.
Making the Right Choice for Your Goal
If your primary focus is Product Selectivity (Ethylene/Propylene):
- Prioritize a furnace with high thermal stability to maintain the reaction strictly between 500°C and 600°C, minimizing non-selective thermal cracking.
If your primary focus is Catalyst Longevity:
- Ensure the system includes robust PID control and atmosphere management (inert gases) to prevent oxidation and structural degradation of active sites.
Reliable data in catalytic cracking is rarely about the highest temperature, but rather the most stable one.
Summary Table:
| Factor | Impact of Programmable Control | Resulting Benefit |
|---|---|---|
| Reaction Selectivity | Maintains 500°C–600°C window | Maximizes high-value olefins (Ethylene/Propylene) |
| Mechanism Control | Favors catalytic over thermal cracking | Reduced non-selective byproducts |
| Coke Management | Prevents temperature spikes | Retards carbon fouling and extends catalyst life |
| Material Integrity | PID control prevents overshoots | Preserves catalyst active sites and crystal structure |
Maximize Your Research Precision with KINTEK
Don't let thermal instability compromise your catalytic cracking data. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable for your unique laboratory needs. Whether you are optimizing olefin selectivity or testing catalyst durability, our programmable high-temp furnaces provide the stability your experiments demand.
Ready to elevate your lab's performance? Contact us today to find your custom furnace solution.
Visual Guide
References
- Optimization of Operational Parameters for Improved Light Olefin Production in Gasoil Cracking over HZSM-5 Catalyst: Temperature and Catalyst Loading Weight as Key Parameters. DOI: 10.21203/rs.3.rs-7402064/v1
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What is the role of high-temperature calcination in a tube furnace for H-Beta zeolite? Engineer Precision Catalysts
- What is the necessity of using sealed silica tubes in the BCM reduction method? Ensuring High-Purity Synthesis
- How does a tube furnace ensure the modification quality during the synthesis of phenyl-modified carbon nitride (PhCN)?
- How does a tube furnace ensure a controlled reaction environment? Achieve Precise Isothermal Oxidation Results
- What is a drop tube furnace and what is its primary purpose? Master Rapid Thermal Processing for Particle Studies
- What is the role of a tube furnace in the production of primary biochar? Expert Sugarcane Pyrolysis Insights
- What role do multi-component mass flow controllers play in tube furnace nitrogen studies? Precise Gas Control for NOx.
- What is the function of a fast-response photoelectric sensor system? Precision Ignition Timing in Tube Furnaces



















