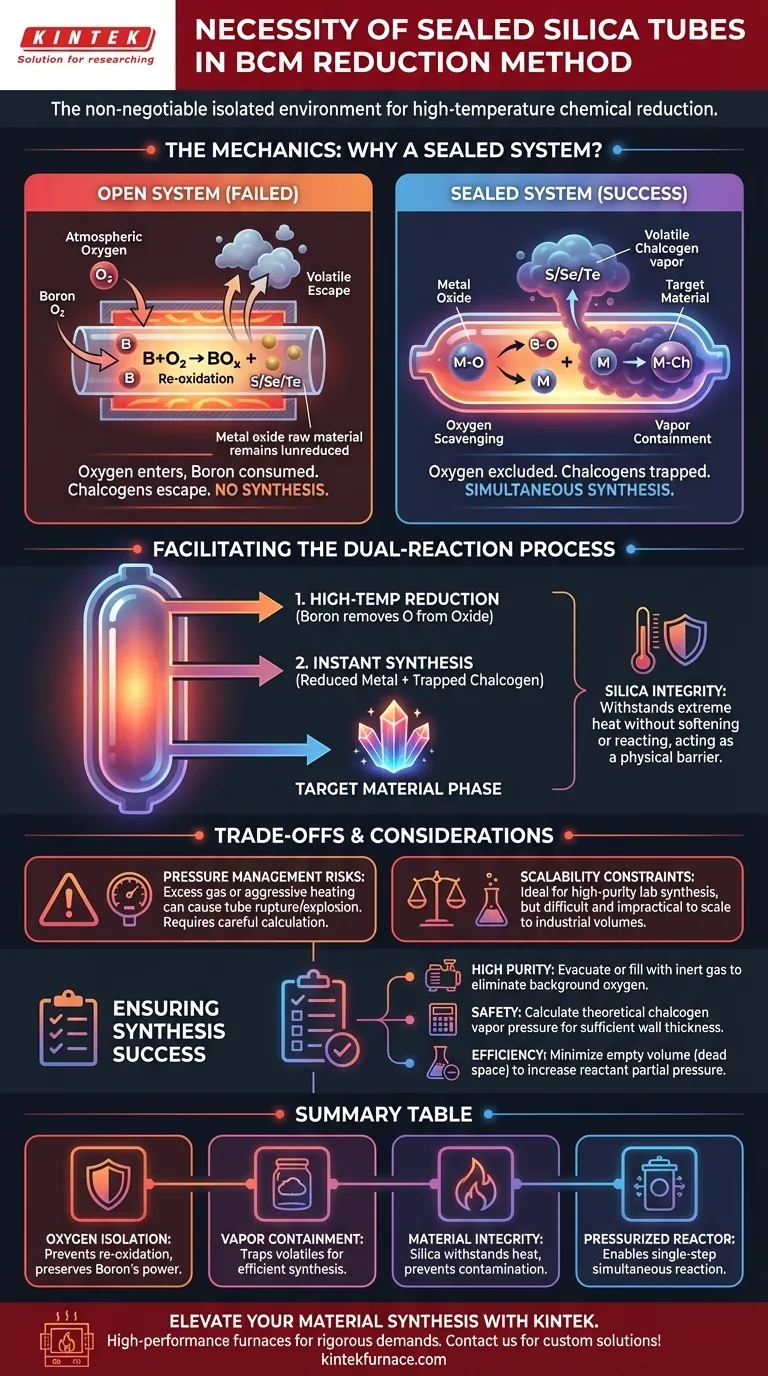
The use of sealed silica tubes is non-negotiable in the Boron-Chalcogen Mixture (BCM) method because they create the isolated environment necessary for high-temperature chemical reduction. Without this closed system, atmospheric oxygen would re-enter the reaction zone, neutralizing the reducing power of boron and preventing the synthesis of the target material.
The sealed silica tube serves as a pressurized, oxygen-free reactor. It forces boron to strip oxygen from raw oxides while simultaneously trapping volatile chalcogens, ensuring they react with the reduced metals to form the final product.

The Mechanics of the Sealed Environment
Preventing Re-oxidation
The primary chemical goal of the BCM method is reduction—removing oxygen from metal oxide raw materials.
Boron acts as the "scavenger," aggressively bonding with oxygen atoms to strip them away from the metal.
If the tube were open to the atmosphere, boron would react with the infinite supply of oxygen in the air rather than the finite oxygen in the raw materials. The seal ensures the reduction process is focused solely on the target oxides.
Containing Volatile Reactants
The high temperatures required for this reaction often exceed the boiling points or sublimation points of chalcogen elements (such as sulfur, selenium, or tellurium).
In an open system, these elements would vaporize and escape the furnace immediately.
The sealed silica tube traps these vapors, maintaining a rich atmosphere of chalcogens that are forced to react with the metals.
Facilitating the Dual-Reaction Process
Simultaneous Reduction and Synthesis
The BCM method is efficient because it combines two steps into one.
As boron removes the oxygen, the metal atoms are left in a reactive, reduced state.
Because the environment is closed, the released chalcogens are immediately available to bond with these exposed metals, forming the target phase instantly.
Maintaining High-Temperature Integrity
Silica is chosen specifically for its ability to withstand the extreme heat required for these reactions without softening or reacting with the sample.
The tube acts as a physical barrier that allows the internal temperature to rise high enough for kinetics to proceed, while chemically isolating the sample from the furnace environment.
Understanding the Trade-offs
Pressure Management Risks
While the sealed environment is necessary, it introduces significant safety considerations regarding internal pressure.
If the reactants produce excess gas, or if the temperature ramp is too aggressive, the vapor pressure inside can exceed the tensile strength of silica.
This can lead to tube rupture or explosion, a common hazard in sealed-tube synthesis.
Scalability Constraints
The necessity of using sealed silica tubes limits the volume of material that can be produced.
This method is ideal for exploratory laboratory synthesis and creating high-purity samples.
However, it is difficult to scale to industrial levels, as creating large, high-pressure sealed silica vessels is chemically and physically impractical.
Ensuring Synthesis Success
If your primary focus is high purity: Ensure the tube is evacuated or filled with inert gas before sealing to eliminate all background atmospheric oxygen.
If your primary focus is safety: Calculate the theoretical vapor pressure of your chalcogen component to ensure the wall thickness of your silica tube is sufficient to withstand the reaction peak.
If your primary focus is reaction efficiency: Minimize the empty volume (dead space) inside the tube to increase the partial pressure of the reactants and drive the kinetics forward.
The sealed silica tube is not just a container; it is an active component of the thermodynamic system that makes the BCM method possible.
Summary Table:
| Feature | Function in BCM Reduction Method | Key Benefit |
|---|---|---|
| Oxygen Isolation | Blocks atmospheric oxygen from entering the reaction | Prevents re-oxidation and preserves boron's reducing power |
| Vapor Containment | Traps volatile chalcogens (S, Se, Te) within the tube | Ensures high partial pressure and efficient material synthesis |
| Material Integrity | High-purity silica withstands extreme heat | Prevents contamination and vessel softening during high-temp cycles |
| Pressurized Reactor | Creates a closed thermodynamic system | Enables simultaneous reduction and synthesis in a single step |
Elevate Your Material Synthesis with KINTEK
Precision is paramount in high-temperature chemical reduction. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of BCM and other advanced synthesis methods. Whether you need a standard setup or a fully customizable lab furnace for your unique research needs, our technology ensures the thermal stability and control your experiments require.
Ready to optimize your lab's thermal processing? Contact us today to find your custom solution!
Visual Guide
References
- С.А. Новиков, Vladislav V. Klepov. Structural evolution and bonding features of electron deficient copper chalcogenides. DOI: 10.1039/d5ce00479a
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- How is heat transferred to the materials inside the tube furnace? Master Uniform Heating for Your Lab
- What is the role of programmed temperature control in a tube furnace? Optimize N-GC-X Catalyst Synthesis
- What are the methods for treating wastewater using a tube furnace? Explore Specialized Thermal Applications
- What are the advantages of horizontal tube furnaces? Achieve Superior Thermal Uniformity and Flexibility
- Why is environment control within a high-temperature tube furnace essential for converting silica into SiNQ?
- What design features contribute to the durability and safety of modern lab tube furnaces? Ensuring Long-Term Reliability and Operator Protection
- How does a high-temperature tube furnace facilitate sulfur melt-diffusion? Precision Heating for PCFC/S Cathodes
- What critical physical environment does a tube furnace provide for iron ore? Master Precision Reduction Control



















