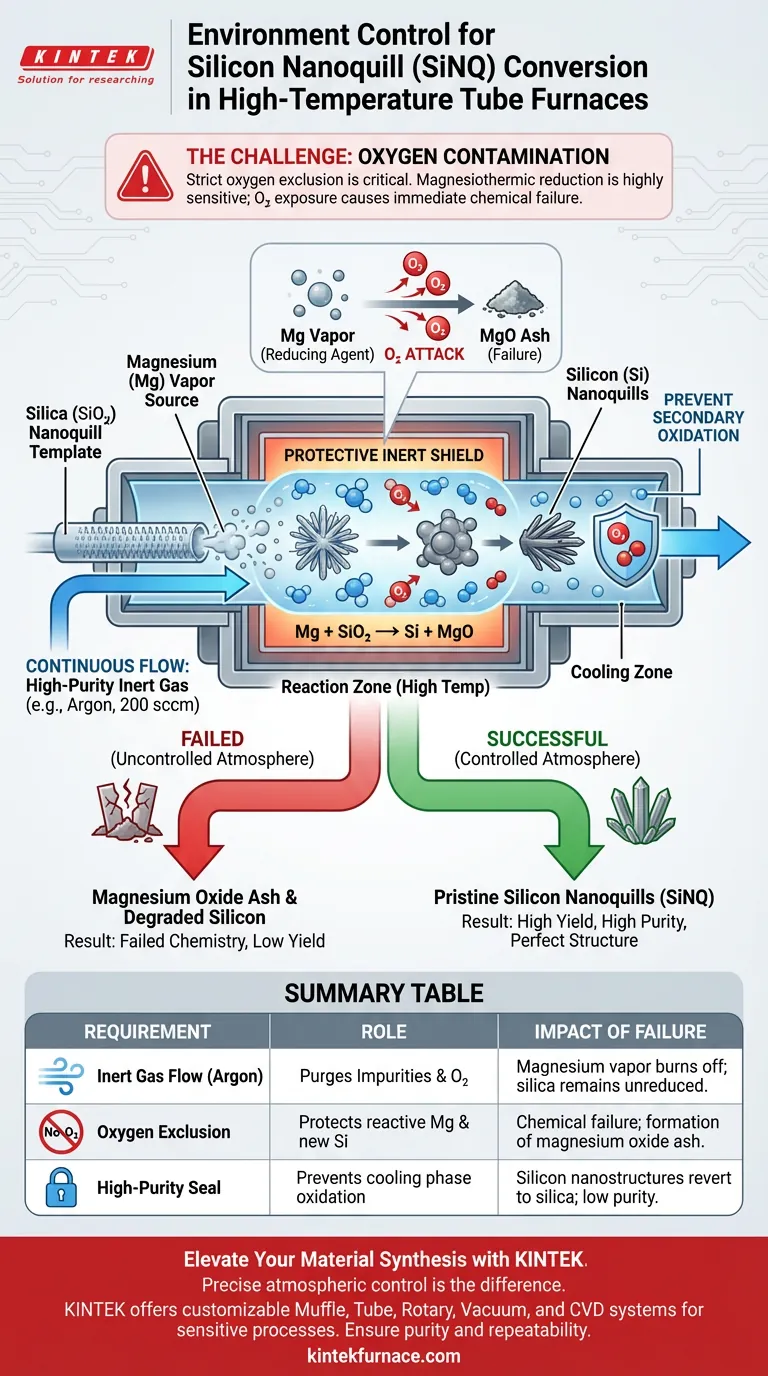
Strict environment control is the only way to prevent chemical failure during conversion. To convert silica nanoquills into silicon nanoquills (SiNQ), the system relies on a magnesiothermic reduction reaction which is highly sensitive to oxygen. A high-temperature tube furnace is essential because it maintains a continuous flow of high-purity inert gas, such as argon, to shield both the reactive magnesium vapor and the newly formed silicon from instantaneous oxidation.
The success of the reduction reaction hinges entirely on excluding oxygen. Without a strictly controlled inert atmosphere, the magnesium reducing agent effectively burns off before it can convert the silica, and any resulting silicon immediately degrades.

The Mechanics of Magnesiothermic Reduction
The Role of Magnesium Vapor
The conversion process uses magnesium vapor as the primary reducing agent.
To transform the silica ($SiO_2$) template into silicon, the magnesium must physically interact with the silica at high temperatures.
The Vulnerability of the Reducing Agent
Magnesium vapor is highly susceptible to oxidation at the elevated temperatures required for this reaction.
If the environment is not controlled, the magnesium reacts with atmospheric oxygen rather than the silica.
This depletes the reducing agent, resulting in magnesium oxide ash rather than the desired silicon nanostructures.
Protecting the Final Product
Preventing Secondary Oxidation
The danger does not end once the silicon is formed.
Newly created silicon nanostructures are chemically active and prone to secondary oxidation.
If exposed to oxygen while still hot, the silicon nanoquills will revert to silica or form impure oxides, ruining the conversion effort.
Ensuring Material Purity
The purity of the final SiNQ product is directly linked to the quality of the atmosphere in the furnace.
By utilizing a tube furnace to maintain a high-purity inert atmosphere, you ensure that the crystal structure remains uncompromised by contaminants.
Operational Criticalities and Pitfalls
The Necessity of Continuous Flow
A static inert environment is often insufficient for this specific reaction.
The primary requirement is a continuous flow of inert gas, such as 200 sccm of argon.
This dynamic flow actively purges any impurities that might outgas during the heating process, maintaining a pristine reaction zone.
Thermal Precision vs. Atmospheric Control
While tube furnaces are praised for thermal stability—such as maintaining precise annealing temperatures to optimize crystallinity—temperature alone cannot drive this conversion.
Operators often make the mistake of focusing on the heat profile while neglecting the gas seal integrity.
Without the inert gas shield, even the most precise thermal profile will result in failed chemistry.
Making the Right Choice for Your Goal
To ensure a successful conversion of silica to silicon nanoquills, apply the following principles:
- If your primary focus is Reaction Yield: Prioritize a robust, continuous flow of argon (e.g., 200 sccm) to ensure the magnesium vapor is consumed by the silica, not by background oxygen.
- If your primary focus is Product Purity: Ensure the tube furnace seals are impeccable to prevent secondary oxidation of the silicon nanostructures during the cooling phase.
Control the atmosphere, and you control the chemistry; neglect it, and the reduction will fail.
Summary Table:
| Requirement | Role in SiNQ Conversion | Impact of Failure |
|---|---|---|
| Inert Gas Flow | Argon (200 sccm) purges impurities and prevents O2 ingress. | Magnesium vapor burns off; silica remains unreduced. |
| Oxygen Exclusion | Protects reactive magnesium vapor and new silicon surfaces. | Chemical failure; formation of magnesium oxide ash. |
| High-Purity Seal | Prevents secondary oxidation during the cooling phase. | Silicon nanostructures revert to silica; low purity. |
| Thermal Precision | Optimizes crystallinity through controlled annealing. | Poor material structure and inconsistent nanostructures. |
Elevate Your Material Synthesis with KINTEK
Precise atmospheric control is the difference between chemical success and failure. KINTEK provides high-performance tube furnaces specifically designed for sensitive processes like magnesiothermic reduction. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to your laboratory's unique high-temperature needs.
Don't let oxygen contamination compromise your silicon nanoquill yields. Ensure material purity and process repeatability with our industry-leading thermal solutions.
Contact our experts today to find your custom furnace solution
Visual Guide
References
- Nancy Chen, Srikanth Pilla. Bioderived silicon nano-quills: synthesis, structure and performance in lithium-ion battery anodes. DOI: 10.1039/d4gc00498a
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What are the different types of tube furnaces available? Find the Perfect Fit for Your Lab's Needs
- Why is a high-temperature tube furnace utilized for the calcination of nano-zinc oxide? Master Microstructure Control
- How do horizontal furnaces contribute to cost savings in industrial processes? Boost Efficiency & Cut Costs
- What are the benefits of quartz tube furnaces? Achieve Purity and Visibility in High-Temp Processes
- What are the technical advantages of using a horizontal tube furnace for the slow pyrolysis of cotton stalks?
- What are the advantages of microwave heating tube furnaces? Achieve Fast, Uniform, and Efficient Material Processing
- Why is a Quartz Tube Furnace with Gas Flow Control Required for Iodine Doping? Precision Single-Atom Catalyst Synthesis
- What role does a tube resistance furnace play in AZO thin film production? Master Pre-Annealing for Perfect Layers



















