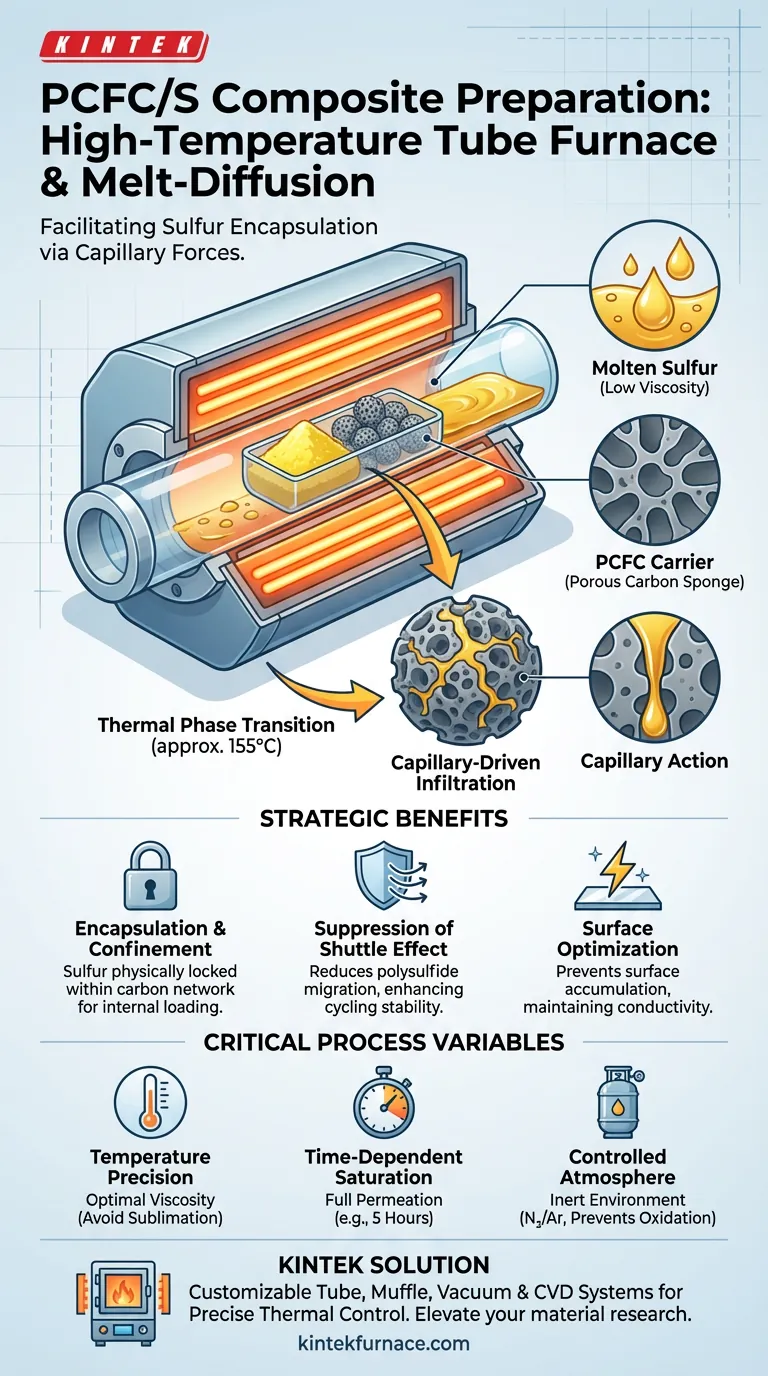
The primary function of a high-temperature tube furnace in this context is to create a precisely controlled thermal environment that heats sulfur above its melting point, typically around 155 °C.
In this molten state, liquid sulfur creates low viscosity, allowing capillary forces to draw it deep into the porous structure of the Porous Carbon (PCFC) carrier. This results in the uniform encapsulation of sulfur within the carbon skeleton, rather than it merely sitting on the surface.
Core Insight: The tube furnace does not simply melt the sulfur; it facilitates a physical interaction where the carbon carrier acts like a sponge. This physical confinement is the essential mechanism for suppressing the "shuttle effect" and ensuring the electrochemical stability of Lithium-Sulfur batteries.

The Mechanics of Melt-Diffusion
Thermal Phase Transition
The tube furnace must maintain a temperature of approximately 155 °C. At this specific thermal plateau, sulfur transitions from a solid to a liquid phase with optimal viscosity for infiltration.
Capillary-Driven Infiltration
Once molten, the sulfur does not spontaneously coat the carbon. Instead, the highly developed pore structure of the PCFC creates capillary pressure.
This pressure actively sucks the liquid sulfur into the internal micro-pores and meso-pores of the carbon matrix.
Controlled Atmosphere
While heat is the driver, the tube furnace also provides a sealed environment.
This allows the process to occur under an inert atmosphere (often utilizing nitrogen or argon), preventing the sulfur from reacting with oxygen or moisture during the extended heating period (often up to 5 hours).
Strategic Benefits for PCFC/S Composites
Encapsulation and Confinement
The primary goal of this process is internal loading.
By driving sulfur into the internal pores, the furnace ensures that sulfur is physically confined within the conductive carbon network.
Suppression of the Shuttle Effect
One of the biggest challenges in Lithium-Sulfur batteries is the "shuttle effect," where polysulfides dissolve and migrate, causing capacity loss.
Melt-diffusion locks the sulfur inside the PCFC pores, significantly reducing this migration and enhancing cycling stability.
Surface Optimization
Proper melt-diffusion prevents surface accumulation.
If sulfur solidifies on the exterior of the carbon particle, it blocks ion transport and reduces conductivity. The tube furnace ensures the exterior surface remains conductive while the active sulfur is stored internally.
Critical Process Variables
Temperature Precision
Control is paramount. If the temperature is too low, the sulfur remains solid or too viscous to penetrate the pores.
If the temperature fluctuates significantly above the target, you risk excessive sublimation or vaporization of the sulfur, leading to loss of active material and inconsistent loading ratios.
Time-Dependent Saturation
Infiltration is not instantaneous.
The furnace must hold the target temperature for a specific duration (e.g., 5 hours) to allow sufficient time for the sulfur to fully permeate the deepest pores of the carbon structure.
Making the Right Choice for Your Goal
When configuring your tube furnace profiles for PCFC/S preparation, consider your specific electrochemical targets:
- If your primary focus is maximizing energy density: Prioritize extended dwell times to ensure the maximum possible volume of sulfur is drawn into the internal pore volume.
- If your primary focus is cycle life (stability): Ensure strictly inert atmospheric control to prevent impurity formation that could degrade the protective confinement of the carbon shell.
Success in this process relies on utilizing the tube furnace not just as a heater, but as a tool to leverage capillary physics for perfect material integration.
Summary Table:
| Process Phase | Key Mechanism | Role of Tube Furnace |
|---|---|---|
| Thermal Transition | Solid to Liquid Phase | Maintains 155°C for optimal sulfur viscosity |
| Infiltration | Capillary-Driven Suction | Provides steady heat to drive sulfur into micro-pores |
| Atmosphere Control | Inert Gas Protection | Prevents oxidation and ensures chemical purity |
| Encapsulation | Physical Confinement | Ensures uniform internal loading and surface conductivity |
Elevate Your Material Research with KINTEK
Precise thermal control is the difference between surface coating and true pore encapsulation. KINTEK provides industry-leading Tube, Muffle, Vacuum, and CVD systems designed to meet the rigorous demands of battery material synthesis.
Backed by expert R&D and manufacturing, our high-temp furnaces are fully customizable to your specific research needs—ensuring consistent, high-performance results for PCFC/S composites and beyond.
Ready to optimize your melt-diffusion process? Contact our technical experts today for a custom solution.
Visual Guide
References
- Ying Liu, Jou‐Hyeon Ahn. Redox‐Active Interlayer with Gradient Adsorption and Catalytic Conversion Functionality for High‐Sulfur‐Loading Lithium‐Sulfur Batteries. DOI: 10.1002/sstr.202500178
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What is the primary function of introducing high-purity argon into the tube furnace? Expert Pyrolysis Solutions
- What are the main advantages of using a tube furnace? Achieve Precise Thermal Control for Your Lab
- What types of atmospheres can be used in a rotary tube furnace? Optimize Your Material Processing with Precision Control
- What are the benefits of induction heating tube furnaces? Unlock Speed, Precision, and Efficiency
- What heating temperatures can tube furnaces achieve? Unlock Precision Up to 1800°C for Your Lab
- What role does a high-vacuum tube furnace (CVD) play in HEA@CNT synthesis? Master Nanocomposite In-Situ Growth
- What critical role does a tube furnace play in the final stage of catalyst preparation for FeOx@KCC-1? Expert Insights
- How does a multi-zone furnace work? Achieve Personalized Comfort and Energy Efficiency



















