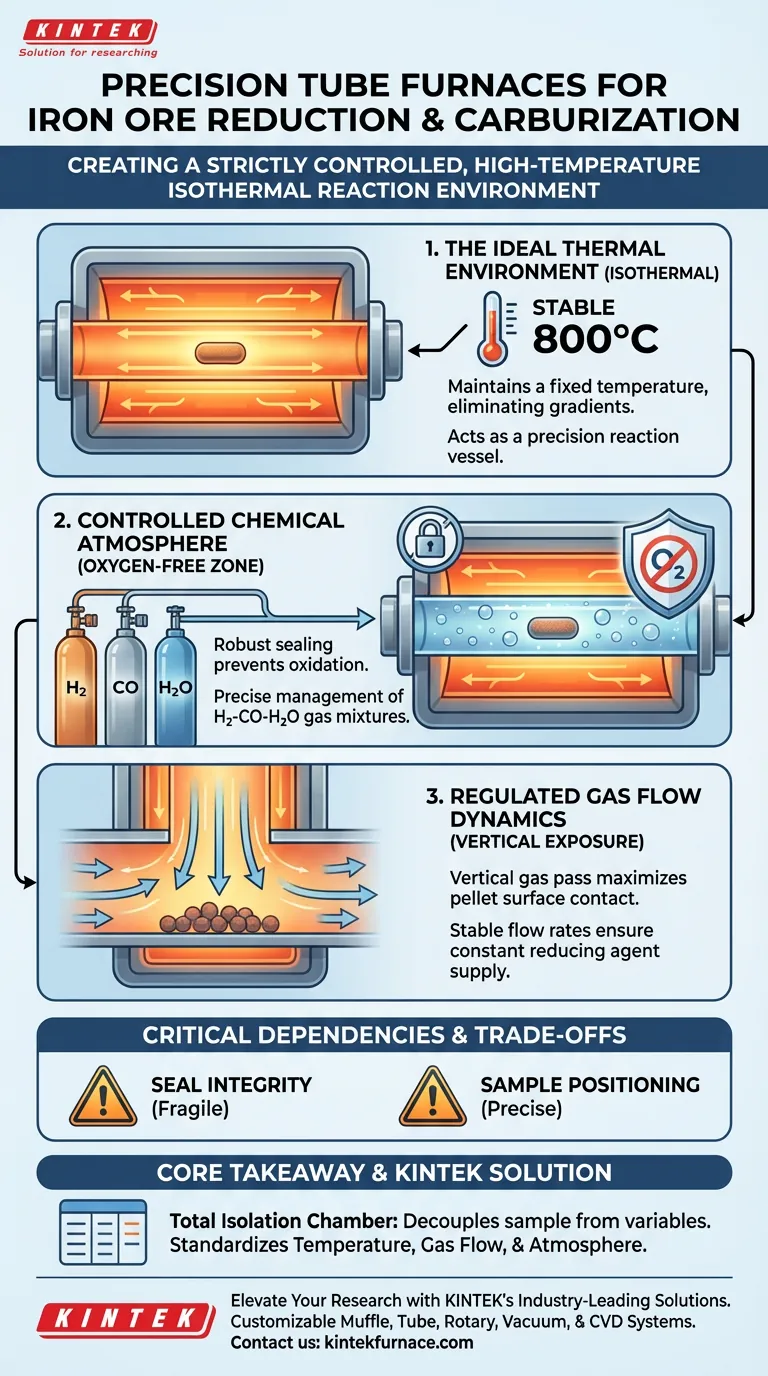
A tube furnace acts as a precision reaction vessel that creates a strictly controlled, high-temperature isothermal environment. For iron ore pellet reduction and carburization, it maintains stable temperatures (such as 800°C) while ensuring reducing gases pass vertically over the pellet surfaces at a consistent flow rate. Most critically, its sealing system establishes an oxygen-free zone, allowing for precise management of H2-CO-H2O gas mixtures without the risk of accidental oxidation.
Core Takeaway: The tube furnace functions as a total isolation chamber, decoupling the sample from external variables. It simultaneously standardizes three critical variables—temperature, gas flow dynamics, and atmospheric composition—to ensure that observed changes in the iron ore are purely the result of the intended chemical reactions.

Creating the Ideal Thermal Environment
The Necessity of Isothermal Conditions
To study reduction and carburization accurately, the sample must be subjected to a consistent thermal profile.
The tube furnace provides an isothermal environment, maintaining a fixed temperature (e.g., 800°C) throughout the experiment. This uniformity prevents temperature gradients that could skew reaction rates across different parts of the pellet.
Functioning as a Reaction Vessel
The furnace does not merely heat the sample; it physically houses the reaction.
It serves as the primary reaction vessel, containing the iron ore pellets within a defined heating zone. This containment is essential for stabilizing the immediate surroundings of the sample.
Controlling the Chemical Atmosphere
Ensuring an Oxygen-Free Zone
One of the most critical functions of the tube furnace is preventing contamination.
The furnace employs a robust sealing system to create an oxygen-free environment. This prevents accidental oxidation, which would reverse or interfere with the reduction process.
Managing Gas Partial Pressures
The environment allows for the precise introduction of specific gas mixtures.
Researchers can control the partial pressures of H2-CO-H2O gas mixtures within the sealed tube. This precise atmospheric composition is what drives the reduction and carburization reactions under experimental conditions.
Regulating Gas Flow Dynamics
Vertical Gas Exposure
The physical orientation of the gas flow is a key environmental feature.
The furnace is designed to ensure that reducing gases pass vertically over the pellet surfaces. This maximizes contact between the reactant gases and the solid iron ore.
Maintaining Stable Flow Rates
Consistency in gas delivery is just as important as the gas composition.
The system creates an environment where the flow rate remains stable throughout the experiment. This ensures that the supply of reducing agents is constant, eliminating flow fluctuations as a variable.
Critical Dependencies and Trade-offs
The Fragility of the Seal
The entire efficacy of the tube furnace environment hinges on the integrity of the sealing system.
If the seal is compromised, the oxygen-free status is lost immediately. Even a minor leak introduces external oxygen, invalidating the control over partial pressures and ruining the data.
Positioning Requirements
The requirement for vertical gas flow imposes specific physical constraints on sample placement.
Pellets must be positioned precisely to align with the vertical gas stream. Incorrect loading can disrupt the intended flow dynamics, leading to uneven reaction exposure on the pellet surfaces.
Ensuring Experimental Success
To leverage the tube furnace effectively for your iron ore experiments, consider these priorities:
- If your primary focus is Reaction Kinetics: Ensure the flow rate is stabilized and the pellets are aligned vertically to guarantee uniform gas exposure.
- If your primary focus is Chemical Purity: Prioritize the integrity of the sealing system to maintain strict partial pressures and prevent accidental oxidation.
The tube furnace is the foundational tool that transforms a chaotic heating process into a controlled scientific experiment.
Summary Table:
| Environmental Factor | Role in Experiment | Experimental Benefit |
|---|---|---|
| Thermal Profile | Isothermal Environment | Eliminates temperature gradients and skewed reaction rates |
| Atmospheric Control | Oxygen-Free Sealing | Prevents accidental oxidation; manages H2-CO-H2O partial pressures |
| Gas Dynamics | Vertical Flow Path | Maximizes gas-to-pellet contact for uniform chemical reaction |
| Flow Regulation | Stable Flow Rates | Ensures constant supply of reducing agents without fluctuations |
Elevate Your Metallurgical Research with KINTEK
Precise control over temperature and atmosphere is the difference between a failed experiment and a scientific breakthrough. KINTEK provides industry-leading laboratory solutions, including Muffle, Tube, Rotary, Vacuum, and CVD systems, all engineered to meet the rigorous demands of iron ore reduction and carburization studies.
Backed by expert R&D and world-class manufacturing, our high-temperature furnaces are fully customizable to your unique research parameters. Don't let atmospheric leaks or thermal instability compromise your data.
Contact KINTEK today to discuss your custom furnace needs and see how our precision engineering can enhance your laboratory’s efficiency and accuracy.
Visual Guide
References
- Effect of Water Vapor on the Reduction and Carburization of Iron Ore Pellets: Theoretical and Experimental Approaches. DOI: 10.1007/s11663-025-03745-y
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What materials are used for the tubes in a High Temperature Tube Furnace? Choose the Right Tube for Your Lab
- How does atmosphere control in a laboratory tube furnace affect Boron Carbide powders? Optimize Your Surface Chemistry
- What is the role of a dual-temperature zone tube furnace in MoS2 CVD growth? Mastering Precision 2D Synthesis
- Why is a controlled nitrogen atmosphere essential during the high-temperature processing of biomass in a tube furnace?
- What critical conditions do laboratory tube furnaces provide for VLS growth of ZnO nanowires? Master Nanoscale Synthesis
- How are tubular furnaces used in industrial and small-batch production? Unlock Controlled Heat for Precision Results
- What is the purpose of flushing a tube furnace with high-purity argon for hours? Ensure Pure Silicon Steel Results
- In what scenarios are laboratory high-temperature tube furnaces or muffle furnaces utilized? Study MgTiO3-CaTiO3 Ceramics



















